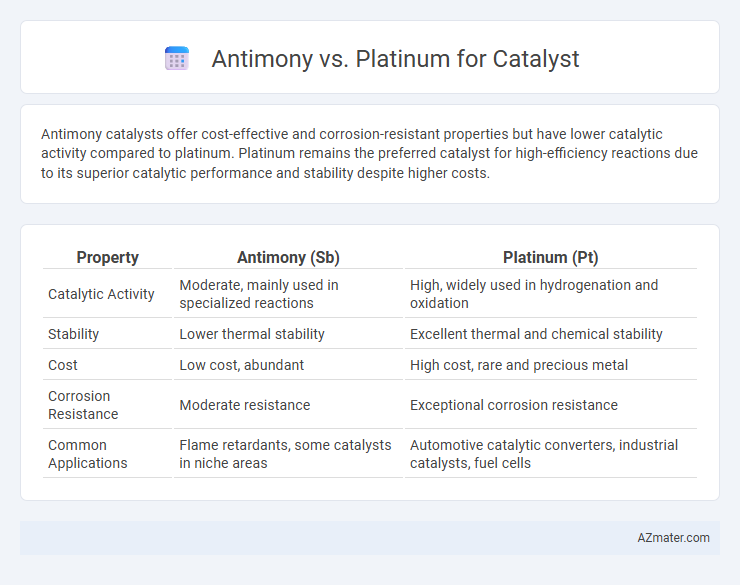
Antimony catalysts offer cost-effective and corrosion-resistant properties but have lower catalytic activity compared to platinum. Platinum remains the preferred catalyst for high-efficiency reactions due to its superior catalytic performance and stability despite higher costs.
Table of Comparison
| Property | Antimony (Sb) | Platinum (Pt) |
|---|---|---|
| Catalytic Activity | Moderate, mainly used in specialized reactions | High, widely used in hydrogenation and oxidation |
| Stability | Lower thermal stability | Excellent thermal and chemical stability |
| Cost | Low cost, abundant | High cost, rare and precious metal |
| Corrosion Resistance | Moderate resistance | Exceptional corrosion resistance |
| Common Applications | Flame retardants, some catalysts in niche areas | Automotive catalytic converters, industrial catalysts, fuel cells |
Introduction to Antimony and Platinum as Catalysts
Antimony and platinum serve distinct roles as catalysts in industrial applications, with platinum being widely valued for its exceptional catalytic activity and stability in processes like hydrogenation and automotive catalytic converters. Antimony, though less common, offers unique catalytic properties in oxidation reactions and is often used to enhance the performance of other catalyst materials. The choice between antimony and platinum hinges on factors such as reaction type, cost efficiency, and desired selectivity in chemical processes.
Chemical Properties and Reactivity
Antimony exhibits moderate catalytic activity due to its p-block element characteristics, with a tendency to form stable oxides that influence its reactivity in oxidation-reduction reactions. Platinum, a transition metal, demonstrates superior catalytic properties attributed to its d-orbitals facilitating adsorption and activation of reactants, particularly in hydrogenation and oxidation processes. The chemical inertness and high resistance to poisoning make platinum catalysts highly efficient, whereas antimony catalysts are more selective but less active under similar reaction conditions.
Historical Applications in Catalysis
Antimony has been historically utilized as a catalyst modifier in the production of polyethylene terephthalate (PET), improving polymerization efficiency and thermal stability. Platinum, known for its exceptional catalytic properties, has played a critical role in automotive catalytic converters since the 1970s, effectively reducing harmful emissions by facilitating oxidation and reduction reactions. The distinct catalytic applications of antimony and platinum highlight their importance in industrial processes, where antimony aids in polymer synthesis and platinum excels in environmental catalysis.
Comparative Catalytic Efficiency
Platinum exhibits superior catalytic efficiency compared to antimony due to its exceptional ability to facilitate hydrogenation and oxidation reactions with higher turnover frequencies and lower activation energies. Antimony, while showing catalytic activity in selective oxidation and as a promoter in certain reactions, generally possesses lower intrinsic catalytic activity and stability under harsh reaction conditions. The distinct electronic structure and surface properties of platinum contribute to its widespread use in catalysis, outperforming antimony in reaction rate and durability metrics.
Cost and Availability
Antimony offers a significantly lower cost compared to platinum, with antimony prices averaging around $5 per kilogram while platinum costs approximately $30,000 per kilogram. Availability of antimony is higher due to its more abundant reserves and stable mining production, unlike platinum, which is rarer and primarily sourced from limited regions such as South Africa and Russia. Cost efficiency and accessibility make antimony a promising alternative to platinum in catalytic applications, especially for large-scale or cost-sensitive industries.
Environmental Impact
Antimony-based catalysts often raise environmental concerns due to their toxicity and potential for bioaccumulation, leading to soil and water contamination risks. Platinum catalysts, despite being rare and expensive, exhibit higher catalytic efficiency and recyclability, resulting in lower emissions and reduced environmental footprint during industrial processes. Transitioning to platinum-supported catalysts supports cleaner production technologies and aligns with stricter environmental regulations on hazardous materials.
Industrial Use Cases
Antimony serves as a cost-effective catalyst additive in the production of polyethylene terephthalate (PET) by enhancing antimony trioxide's catalytic efficiency for polymerization reactions. Platinum, prized for its exceptional catalytic activity and resistance to poisoning, is extensively used in automotive catalytic converters and chemical manufacturing processes such as hydrogenation and oxidation reactions. Industrial applications favor platinum for its durability and reusability despite its high cost, whereas antimony is preferred for large-scale polymer production due to its affordability and sufficient catalytic performance.
Stability and Longevity in Reactions
Antimony-based catalysts exhibit moderate stability but tend to degrade faster under high-temperature and oxidative reaction conditions compared to platinum catalysts. Platinum catalysts maintain superior longevity due to their exceptional resistance to sintering and chemical poisoning, making them ideal for prolonged catalytic processes. The enhanced durability of platinum ensures consistent catalytic performance and reduces the frequency of catalyst replacement in industrial applications.
Future Trends in Catalyst Development
Antimony is gaining attention as a cost-effective alternative to platinum in catalyst development due to its abundant availability and favorable electronic properties. Future trends emphasize the integration of antimony-based catalysts in hydrogen evolution and oxygen reduction reactions, leveraging its potential to enhance catalytic efficiency while reducing reliance on expensive platinum. Research is increasingly directed towards optimizing antimony's structural stability and catalytic activity through alloying and nanostructuring techniques for sustainable energy applications.
Conclusion: Choosing Between Antimony and Platinum
Antimony offers a cost-effective alternative to platinum with respectable catalytic activity in select chemical reactions, especially in oxidation processes. Platinum remains superior in durability, catalytic efficiency, and resistance to poisoning, making it ideal for high-performance applications like automotive catalytic converters and fuel cells. Selection depends on balancing budget constraints with performance requirements, where antimony suits economical, moderate-use scenarios, while platinum excels in demanding, long-term catalytic environments.
Infographic: Antimony vs Platinum for Catalyst
 azmater.com
azmater.com