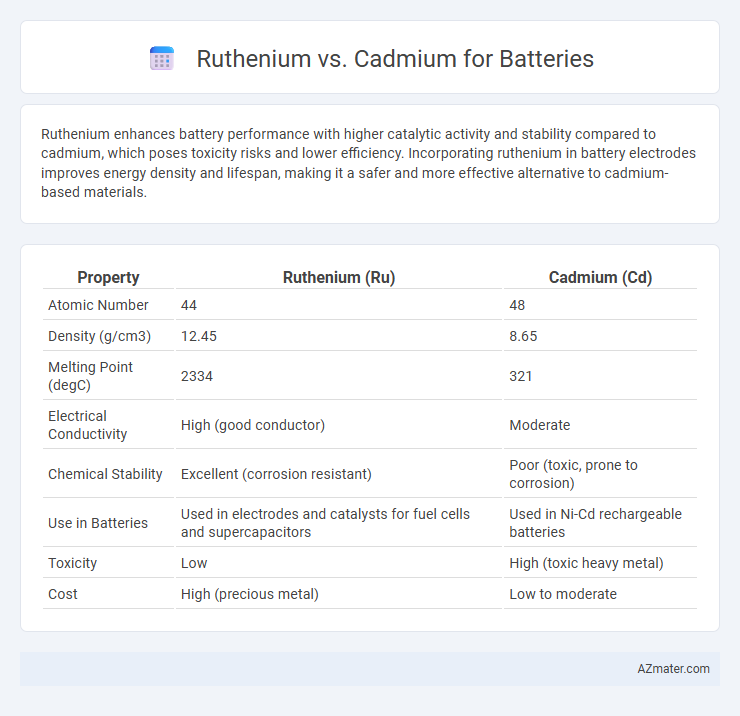
Ruthenium enhances battery performance with higher catalytic activity and stability compared to cadmium, which poses toxicity risks and lower efficiency. Incorporating ruthenium in battery electrodes improves energy density and lifespan, making it a safer and more effective alternative to cadmium-based materials.
Table of Comparison
| Property | Ruthenium (Ru) | Cadmium (Cd) |
|---|---|---|
| Atomic Number | 44 | 48 |
| Density (g/cm3) | 12.45 | 8.65 |
| Melting Point (degC) | 2334 | 321 |
| Electrical Conductivity | High (good conductor) | Moderate |
| Chemical Stability | Excellent (corrosion resistant) | Poor (toxic, prone to corrosion) |
| Use in Batteries | Used in electrodes and catalysts for fuel cells and supercapacitors | Used in Ni-Cd rechargeable batteries |
| Toxicity | Low | High (toxic heavy metal) |
| Cost | High (precious metal) | Low to moderate |
Introduction: Comparing Ruthenium and Cadmium in Battery Technologies
Ruthenium offers exceptional electrochemical stability and high conductivity, making it a promising material for advanced battery electrodes compared to cadmium. Cadmium, historically used in nickel-cadmium (NiCd) batteries, poses significant environmental and toxicity concerns that limit its modern applications. Ruthenium's superior corrosion resistance and efficiency enhance battery lifespan and performance, positioning it as a preferable alternative in cutting-edge energy storage solutions.
Chemical Properties: Ruthenium vs Cadmium
Ruthenium exhibits higher chemical stability and corrosion resistance compared to cadmium, making it advantageous for long-lasting battery electrodes. Cadmium's electrochemical properties allow efficient charge storage but with toxicity concerns limiting its widespread use. Ruthenium's catalytic activity enhances battery charge-discharge cycles, while cadmium's lower cost remains a factor despite environmental drawbacks.
Electrochemical Performance in Batteries
Ruthenium exhibits superior electrochemical performance in batteries due to its high electrical conductivity, excellent catalytic activity, and robust cycling stability, making it ideal for enhancing charge-discharge efficiency and lifespan in lithium-ion and sodium-ion batteries. Cadmium, while offering moderate conductivity, suffers from lower electrochemical stability and environmental toxicity, limiting its practical application in high-performance rechargeable batteries. Ruthenium's ability to facilitate faster electron transfer and maintain structural integrity under prolonged cycling significantly outperforms cadmium-based electrode materials in energy density and rate capability.
Energy Density and Efficiency Analysis
Ruthenium-based electrodes exhibit higher energy density and improved cycling efficiency compared to cadmium in battery applications, attributed to ruthenium's superior electrochemical stability and conductivity. Cadmium batteries, such as NiCd, provide moderate energy density but face challenges with memory effect and efficiency degradation over time. Advanced ruthenium composites enhance battery life and charge retention, making them more suitable for high-performance energy storage solutions.
Environmental Impact of Ruthenium and Cadmium
Ruthenium, a rare transition metal, exhibits lower environmental toxicity compared to cadmium, which is a heavy metal known for its high toxicity and persistence in ecosystems. Cadmium's use in batteries poses significant risks such as soil and water contamination, bioaccumulation, and adverse health effects in humans, leading to strict regulatory restrictions. Ruthenium-based electrodes offer improved recyclability and reduced ecological hazards, making them a more sustainable option for battery applications.
Safety Considerations in Battery Applications
Ruthenium exhibits superior safety characteristics in battery applications due to its high thermal stability and resistance to dendrite formation, which reduces the risk of short circuits and thermal runaway. In contrast, cadmium-based batteries pose significant safety concerns as cadmium is toxic and prone to leakage, increasing environmental and health hazards during usage and disposal. Selecting ruthenium for electrode materials enhances battery longevity while minimizing hazardous incidents linked to chemical toxicity and instability.
Cost and Resource Availability
Ruthenium, a rare and expensive platinum-group metal, significantly increases battery costs due to limited global reserves and complex extraction processes. Cadmium offers a more affordable alternative with abundant availability, but its toxicity and environmental hazards restrict widespread use in modern batteries. Cost-efficiency and resource abundance favor cadmium, while ruthenium's scarcity and high price impact large-scale battery production feasibility.
Scalability for Commercial Battery Production
Ruthenium offers superior scalability for commercial battery production due to its higher electrochemical stability and longer cycle life, enabling consistent performance in large-scale manufacturing. Cadmium, while cheaper, presents significant toxicity and environmental concerns that hinder its widespread adoption and regulatory approval for mass-market batteries. The cost-effectiveness and eco-friendliness of ruthenium-based cathodes make them more viable for sustainable, scalable commercial battery solutions.
Emerging Research and Innovations
Emerging research on ruthenium and cadmium for battery applications highlights ruthenium's superior catalytic properties and high conductivity, enhancing energy density and charge-discharge rates in lithium-ion and emerging sodium-ion batteries. Innovations in ruthenium-based electrode materials demonstrate increased durability and thermal stability compared to cadmium, which faces environmental and toxicity concerns limiting its commercial viability. Recent studies focus on ruthenium nanostructures and their integration with solid electrolytes to achieve longer cycle life and safer, high-performance batteries.
Future Prospects: Ruthenium or Cadmium for Next-Gen Batteries
Ruthenium holds significant promise for next-generation batteries due to its exceptional conductivity and stability, enabling higher energy density and faster charge cycles compared to cadmium. Cadmium, while historically used in nickel-cadmium batteries, faces environmental and toxicity concerns that limit its future viability in sustainable energy storage solutions. Advances in ruthenium-based catalysts and electrode materials are driving research towards more efficient, durable, and eco-friendly batteries for electric vehicles and grid storage.
Infographic: Ruthenium vs Cadmium for Battery
 azmater.com
azmater.com