Magnesium offers lightweight and excellent corrosion resistance, making it ideal for chemical equipment requiring durability and cost-efficiency. Platinum provides superior chemical inertness and high-temperature stability, essential for equipment handling highly reactive or corrosive substances.
Table of Comparison
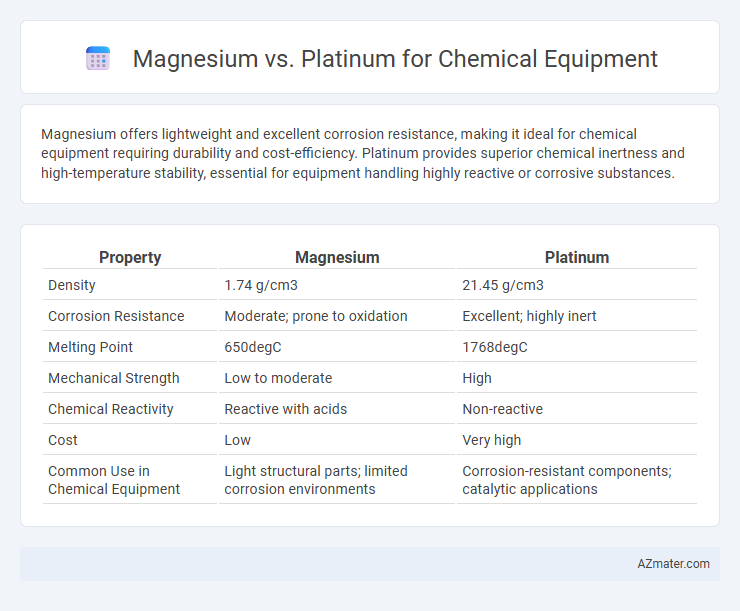
| Property | Magnesium | Platinum |
|---|---|---|
| Density | 1.74 g/cm3 | 21.45 g/cm3 |
| Corrosion Resistance | Moderate; prone to oxidation | Excellent; highly inert |
| Melting Point | 650degC | 1768degC |
| Mechanical Strength | Low to moderate | High |
| Chemical Reactivity | Reactive with acids | Non-reactive |
| Cost | Low | Very high |
| Common Use in Chemical Equipment | Light structural parts; limited corrosion environments | Corrosion-resistant components; catalytic applications |
Introduction to Magnesium and Platinum in Chemical Equipment
Magnesium and platinum serve distinct roles in chemical equipment due to their unique properties. Magnesium, valued for its lightweight and excellent thermal conductivity, is commonly used in lightweight structural components and heat exchangers. Platinum, renowned for its exceptional corrosion resistance and catalytic properties, is primarily employed as a catalyst and in high-temperature reaction vessels, ensuring durability and efficiency in harsh chemical environments.
Chemical Properties: Magnesium vs Platinum
Magnesium exhibits high reactivity with acids and oxidizing agents, making it prone to corrosion and unsuitable for certain chemical equipment exposed to aggressive substances. Platinum, characterized by its exceptional chemical inertness and resistance to oxidation, maintains stability under harsh chemical environments, proving ideal for catalysts and high-precision equipment. The contrasting chemical properties--magnesium's high reactivity versus platinum's chemical inertness--directly influence their selection in manufacturing chemical containers, reactors, and catalytic components.
Corrosion Resistance and Durability Comparison
Magnesium exhibits lower corrosion resistance compared to platinum, as it is more prone to oxidation and acidic environments, which limits its durability in harsh chemical applications. Platinum's exceptional corrosion resistance and inertness, even in aggressive chemical environments, make it highly durable for long-term use in chemical equipment. Consequently, platinum is preferred over magnesium for applications demanding superior longevity and minimal maintenance in corrosive conditions.
Mechanical Strength and Weight Considerations
Magnesium offers significantly lower weight compared to platinum, making it ideal for applications where reducing overall equipment mass is crucial. However, platinum surpasses magnesium in mechanical strength and corrosion resistance, ensuring greater durability and longevity under extreme chemical environments. Engineers must balance magnesium's lightweight advantage against platinum's superior strength and chemical stability when selecting materials for chemical equipment components.
Cost Analysis: Magnesium vs Platinum
Magnesium offers a significantly lower cost compared to platinum, making it a more economical choice for chemical equipment where budget constraints are critical. Platinum's high price is justified by its exceptional corrosion resistance and catalytic properties, which may reduce long-term maintenance costs despite the initial expense. Evaluating total lifecycle costs, including purchase, durability, and replacement frequency, is essential for optimizing material selection between magnesium and platinum in chemical processing applications.
Thermal Conductivity and Heat Resistance
Magnesium offers excellent thermal conductivity, approximately 156 W/m*K, making it ideal for rapid heat dissipation in chemical equipment. Platinum, with a lower thermal conductivity around 71.6 W/m*K, excels in heat resistance, maintaining structural integrity at temperatures exceeding 1,700degC. Choosing between magnesium and platinum depends on the need for efficient heat transfer versus superior high-temperature stability in chemical processing environments.
Common Applications in Chemical Industry
Magnesium is widely used in chemical equipment for applications requiring lightweight and corrosion-resistant materials, especially in reactors and heat exchangers handling strong alkaline solutions. Platinum, valued for its exceptional catalytic properties and high resistance to corrosion and oxidation, is primarily employed in catalytic converters, electrodes, and sensors within chemical processing plants. While magnesium suits structural components and general corrosion resistance, platinum is preferred for catalytic processes and environments demanding superior chemical inertness.
Environmental Impact and Sustainability
Magnesium exhibits a lower environmental impact compared to platinum due to its abundance and lower energy requirements for extraction and processing. Its recyclability and biodegradability contribute to enhanced sustainability in chemical equipment applications. Platinum, while highly durable and corrosion-resistant, involves more intensive mining processes and limited availability, leading to higher ecological footprints and sustainability concerns.
Safety Factors and Handling Precautions
Magnesium, being highly reactive and flammable, requires rigorous safety measures such as avoiding exposure to moisture and sparks, and using non-sparking tools during handling, whereas platinum is chemically inert and significantly safer to handle with minimal risk of ignition or toxic reactions. Magnesium equipment demands proper storage in dry environments and the use of personal protective equipment (PPE) to prevent burns and fire hazards, while platinum equipment primarily necessitates standard laboratory safety protocols due to its stability. The critical safety consideration for magnesium centers on its potential for spontaneous combustion, contrasting with platinum's excellent corrosion resistance and non-reactivity, making it more suitable for high-temperature chemical processes.
Conclusion: Choosing the Right Material for Chemical Equipment
Magnesium offers lightweight and cost-effective solutions ideal for applications requiring corrosion resistance in mildly reactive environments, while platinum provides superior chemical inertness and durability for highly corrosive or high-temperature reactions despite its higher cost. Selection depends on operational conditions, chemical compatibility, and budget constraints, with platinum favored in critical processes demanding longevity and minimal contamination. Evaluating specific chemical exposure and mechanical requirements ensures optimal material performance and safety in chemical equipment design.
Infographic: Magnesium vs Platinum for Chemical Equipment
 azmater.com
azmater.com