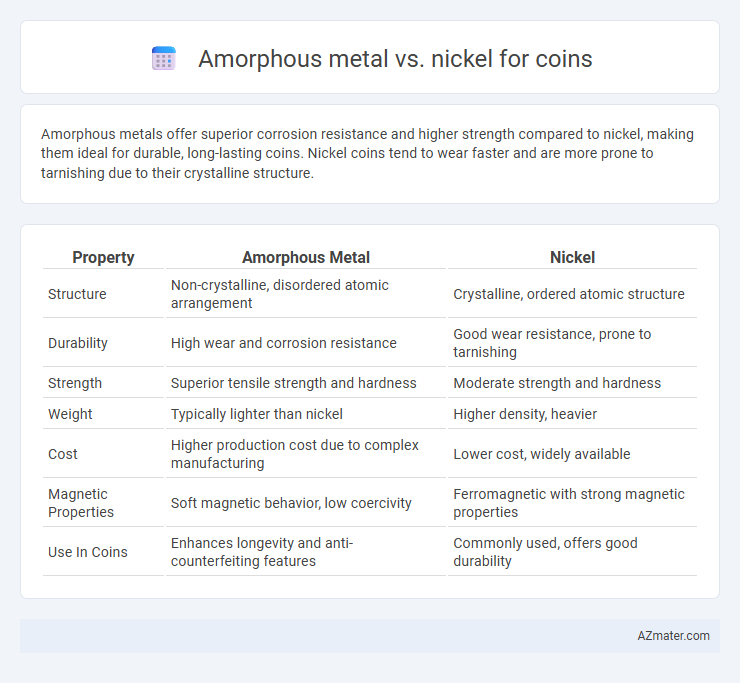
Amorphous metals offer superior corrosion resistance and higher strength compared to nickel, making them ideal for durable, long-lasting coins. Nickel coins tend to wear faster and are more prone to tarnishing due to their crystalline structure.
Table of Comparison
| Property | Amorphous Metal | Nickel |
|---|---|---|
| Structure | Non-crystalline, disordered atomic arrangement | Crystalline, ordered atomic structure |
| Durability | High wear and corrosion resistance | Good wear resistance, prone to tarnishing |
| Strength | Superior tensile strength and hardness | Moderate strength and hardness |
| Weight | Typically lighter than nickel | Higher density, heavier |
| Cost | Higher production cost due to complex manufacturing | Lower cost, widely available |
| Magnetic Properties | Soft magnetic behavior, low coercivity | Ferromagnetic with strong magnetic properties |
| Use In Coins | Enhances longevity and anti-counterfeiting features | Commonly used, offers good durability |
Introduction to Coinage Materials
Amorphous metals exhibit superior corrosion resistance, high strength, and enhanced wear properties compared to traditional nickel used in coinage, making them a promising alternative for durable currency production. Unlike crystalline nickel, amorphous metals lack a defined grain structure, resulting in improved mechanical performance and reduced susceptibility to environmental degradation. These materials offer coin manufacturers increased longevity and security features essential for modern circulation coins.
What Are Amorphous Metals?
Amorphous metals, also known as metallic glasses, are alloys with a disordered atomic structure that differs from the crystalline arrangement found in traditional metals like nickel. Their unique non-crystalline structure gives them exceptional hardness, corrosion resistance, and magnetic properties, making them ideal for durable coins. Compared to nickel, amorphous metals offer greater wear resistance and longer-lasting aesthetic appeal in coinage applications.
Overview of Nickel in Coin Production
Nickel is widely used in coin production due to its excellent corrosion resistance, hardness, and durability, which ensure longevity in circulation. Its natural silvery appearance and ability to form strong alloys with other metals make it ideal for minting coins with high wear resistance and aesthetic appeal. The metal's proven performance in various climates and handling conditions secures its position as a preferred choice in numismatic applications.
Physical Properties: Amorphous Metal vs Nickel
Amorphous metals exhibit a non-crystalline atomic structure, resulting in higher strength, hardness, and improved wear resistance compared to crystalline nickel used in coins. Nickel, with its crystalline lattice, offers excellent corrosion resistance and ductility but tends to be less resilient to deformation under stress. The superior hardness and elasticity of amorphous metals enhance the durability and lifespan of coins, making them less susceptible to scratches and permanent damage during circulation.
Durability and Wear Resistance Comparison
Amorphous metals exhibit superior durability and wear resistance compared to traditional nickel due to their non-crystalline atomic structure, which enhances hardness and reduces susceptibility to deformation and corrosion. Nickel, while commonly used in coins for its corrosion resistance and ease of manufacturing, tends to wear down more rapidly under frequent handling and environmental exposure. The increased wear resistance of amorphous metal extends coin lifespan, making it a more durable alternative for high-circulation currency.
Cost and Economic Feasibility
Amorphous metals typically exhibit higher initial production costs compared to nickel due to advanced manufacturing processes and material composition. Nickel remains economically favorable for coin production because of its abundant supply and established smelting infrastructure, reducing per-unit cost substantially. Cost-efficiency analysis highlights nickel's superiority in large-scale minting operations despite amorphous metals offering enhanced wear resistance.
Manufacturing and Minting Processes
Amorphous metals offer superior wear resistance and corrosion protection in coin manufacturing due to their non-crystalline atomic structure, allowing for longer-lasting dies and sharper minting details compared to traditional nickel. The casting process for amorphous metals requires rapid cooling techniques to maintain their unique structure, contrasting with the standard melting and alloying procedures used for nickel coins. Minting with amorphous metal blanks can reduce maintenance downtime and improve production efficiency, as their enhanced hardness minimizes die degradation during high-volume pressing.
Security Features and Counterfeit Resistance
Amorphous metals exhibit superior security features for coins due to their unique atomic structure, which enhances resistance to wear, corrosion, and magnetic tampering, making them difficult to replicate and detect counterfeit attempts. Nickel, while durable and widely used, lacks the distinct non-crystalline properties of amorphous metals, resulting in higher susceptibility to cloning and conventional counterfeiting techniques. The enhanced hardness and distinctive electromagnetic signatures of amorphous metal coins provide advanced counterfeit resistance compared to traditional nickel-based currency.
Environmental Impact and Sustainability
Amorphous metals offer significant environmental benefits over nickel in coin production due to their lower raw material extraction impact and higher recyclability, reducing landfill waste and mining-related pollution. Nickel mining contributes to habitat destruction and toxic runoff, while amorphous metals, often alloyed with more abundant and less harmful elements, support sustainable resource use. The long lifespan and corrosion resistance of amorphous metal coins decrease replacement frequency, further minimizing environmental footprints compared to traditional nickel-based currency.
Future Prospects in Coin Technology
Amorphous metals, with their superior corrosion resistance and enhanced wear properties, offer promising advancements over traditional nickel for coin production, potentially extending coin lifespan and reducing maintenance costs. The unique atomic structure of amorphous metals provides increased durability and resistance to deformation, which can improve coin security features against counterfeiting. Emerging research in coin technology highlights the feasibility of integrating amorphous metal alloys to create next-generation coins that combine cost-effectiveness with enhanced performance in circulation.
Infographic: Amorphous metal vs Nickel for Coin
 azmater.com
azmater.com