Tin offers excellent corrosion resistance and low toxicity, making it ideal for soldering alloys and plating applications. Silver provides superior electrical conductivity and strength, commonly used in high-performance alloys and electrical contacts.
Table of Comparison
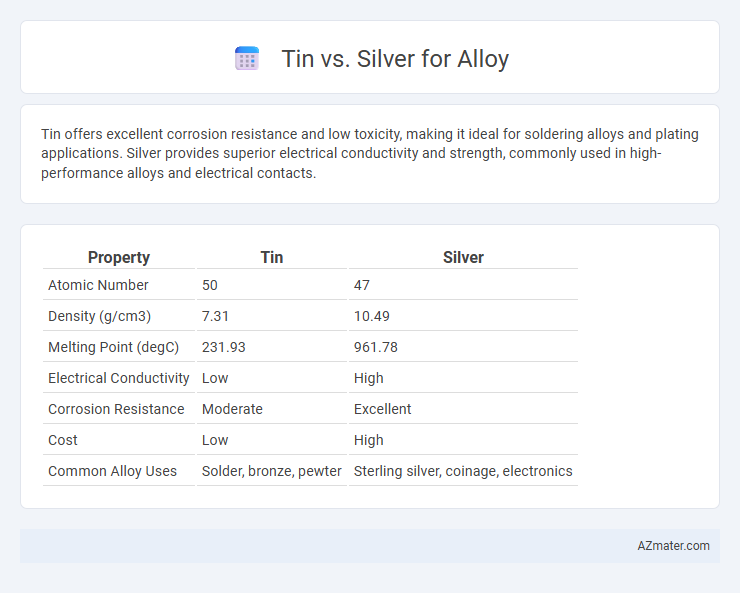
| Property | Tin | Silver |
|---|---|---|
| Atomic Number | 50 | 47 |
| Density (g/cm3) | 7.31 | 10.49 |
| Melting Point (degC) | 231.93 | 961.78 |
| Electrical Conductivity | Low | High |
| Corrosion Resistance | Moderate | Excellent |
| Cost | Low | High |
| Common Alloy Uses | Solder, bronze, pewter | Sterling silver, coinage, electronics |
Introduction to Tin and Silver Alloys
Tin and silver are essential metals in alloy production, prized for their unique properties. Tin is commonly used in alloys like bronze, providing corrosion resistance and malleability, while silver alloys are valued for their high conductivity and aesthetic appeal in jewelry and electronics. Combining tin and silver enhances strength and durability, making these alloys suitable for a wide range of industrial applications.
Chemical Properties of Tin and Silver
Tin exhibits a low melting point of 232 degC and excellent corrosion resistance due to its stable oxide layer, making it ideal for solder and plating alloys. Silver boasts a higher melting point of 962 degC, exceptional electrical and thermal conductivity, and strong antimicrobial properties, enhancing alloy applications requiring conductivity and durability. Both elements chemically form various intermetallic compounds that influence alloy hardness, malleability, and resistance to oxidation.
Melting Points: Tin vs Silver
Tin has a melting point of 231.9degC, making it significantly lower than silver's melting point of 961.8degC. This difference in melting points influences their use in alloys, where tin's lower melting point allows for easier melting and casting at reduced temperatures. Silver alloys benefit from higher melting points, enhancing durability and resistance to heat in applications like jewelry and electronics.
Electrical Conductivity Comparison
Tin exhibits lower electrical conductivity compared to silver, with silver having an exceptional conductivity of approximately 63 x 10^6 S/m, while tin's conductivity is closer to 9.17 x 10^6 S/m. This significant difference makes silver alloys preferable in applications requiring superior electrical performance, such as high-frequency connectors and conductors. However, tin alloys often offer better corrosion resistance and solderability, which can be critical in certain electronic components despite their reduced conductivity.
Corrosion Resistance in Alloys
Tin offers superior corrosion resistance in alloys due to its ability to form a stable, protective oxide layer that prevents further oxidation, making it ideal for coatings and soldering applications. Silver, while providing excellent electrical conductivity and aesthetic appeal, is more susceptible to tarnishing and corrosion when exposed to sulfur compounds and environmental pollutants. Alloys with higher tin content typically exhibit enhanced durability and longevity in harsh environments compared to silver-based alloys.
Tin vs Silver: Mechanical Strength
Tin alloys generally offer lower mechanical strength compared to silver alloys due to tin's softer and more malleable nature. Silver alloys display higher tensile strength and better durability, making them suitable for applications requiring robust mechanical performance. The difference in crystal structure and bonding contributes to silver's superior resistance to deformation under stress compared to tin-based alloys.
Cost and Availability of Tin and Silver
Tin is significantly more affordable and widely available compared to silver, making it a cost-effective choice for alloy production. Silver, while valuable for its superior conductivity and antibacterial properties, is much rarer and commands a higher market price. The abundant global reserves of tin ensure steady supply chains, whereas silver's limited availability can lead to price volatility and supply constraints.
Common Applications of Each Metal
Tin is widely used in alloys such as bronze and solder due to its excellent corrosion resistance and low melting point, making it ideal for coatings, electrical components, and plumbing fixtures. Silver is favored in high-strength alloys for electronics, jewelry, and photographic equipment because of its superior electrical conductivity and antimicrobial properties. Both metals enhance alloy performance but target different industrial applications based on their unique physical and chemical characteristics.
Environmental Impact and Sustainability
Tin alloys typically exhibit a lower environmental footprint compared to silver due to tin's abundance and less intensive mining processes, leading to reduced carbon emissions and habitat disruption. Silver mining often results in significant ecological damage, including water contamination from toxic chemicals used in extraction, posing sustainability challenges. Choosing tin-based alloys supports more sustainable production practices by minimizing ecological harm and promoting resource efficiency in manufacturing.
Choosing the Right Alloy: Tin or Silver
Choosing the right alloy depends on the application's thermal conductivity, corrosion resistance, and cost-effectiveness; tin alloys offer excellent corrosion resistance and solderability, making them ideal for electronics and plumbing. Silver alloys provide superior electrical conductivity, higher strength, and antimicrobial properties, suited for high-performance electrical contacts and jewelry. Evaluating mechanical requirements and environmental exposure ensures selecting tin or silver alloys aligns with durability and performance goals.
Infographic: Tin vs Silver for Alloy
 azmater.com
azmater.com