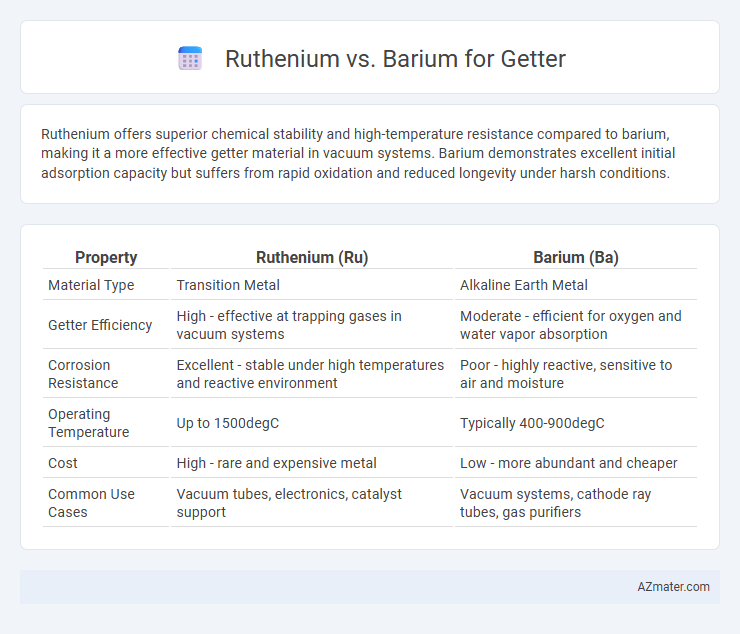
Ruthenium offers superior chemical stability and high-temperature resistance compared to barium, making it a more effective getter material in vacuum systems. Barium demonstrates excellent initial adsorption capacity but suffers from rapid oxidation and reduced longevity under harsh conditions.
Table of Comparison
| Property | Ruthenium (Ru) | Barium (Ba) |
|---|---|---|
| Material Type | Transition Metal | Alkaline Earth Metal |
| Getter Efficiency | High - effective at trapping gases in vacuum systems | Moderate - efficient for oxygen and water vapor absorption |
| Corrosion Resistance | Excellent - stable under high temperatures and reactive environment | Poor - highly reactive, sensitive to air and moisture |
| Operating Temperature | Up to 1500degC | Typically 400-900degC |
| Cost | High - rare and expensive metal | Low - more abundant and cheaper |
| Common Use Cases | Vacuum tubes, electronics, catalyst support | Vacuum systems, cathode ray tubes, gas purifiers |
Introduction to Getter Materials: Ruthenium vs Barium
Getter materials play a critical role in vacuum technology by trapping residual gases to maintain ultra-high vacuum conditions. Ruthenium demonstrates excellent stability and corrosion resistance as a getter, making it suitable for long-lasting applications, while barium offers superior reactive properties that enable rapid gas absorption but with less durability. The choice between ruthenium and barium depends on specific vacuum system requirements, balancing reactivity and longevity for optimal performance.
Chemical Properties and Reactivity
Ruthenium exhibits excellent chemical stability and resistance to oxidation, making it highly effective as a getter in vacuum systems where it rapidly absorbs residual gases such as oxygen and nitrogen. Barium, in contrast, is highly reactive and readily forms stable compounds with oxygen and other gases, providing strong gettering action but with lower thermal stability compared to ruthenium. The choice between ruthenium and barium as getters depends on the application's temperature constraints and the specific gases targeted for absorption.
Efficiency in Gas Absorption
Ruthenium exhibits superior efficiency in gas absorption as a getter compared to barium due to its higher chemical stability and stronger affinity for residual gases such as oxygen, nitrogen, and hydrogen. Barium, while effective at initial gas sorption, tends to oxidize rapidly, reducing its long-term getter performance and necessitating frequent reactivation. The robustness of ruthenium's surface chemistry ensures sustained vacuum conditions, making it more reliable for applications requiring continuous gas absorption.
Application in Vacuum Technology
Ruthenium and Barium serve as critical materials for getters in vacuum technology, with Ruthenium often preferred for its exceptional resistance to oxidation and superior catalytic properties enabling efficient gas absorption. Barium getters exhibit high chemical reactivity, effectively absorbing gases like oxygen and nitrogen in vacuum tubes and cathode ray devices. The choice between Ruthenium and Barium depends on the specific vacuum application requirements, where Ruthenium-based getters offer longevity and stability, while Barium getters provide rapid gas absorption in dynamic vacuum environments.
Stability Under Operating Conditions
Ruthenium exhibits superior stability under operating conditions compared to barium when used as a getter, maintaining its efficacy in high-temperature and reactive environments. Barium, while effective at sorbing gases, tends to oxidize and degrade more rapidly, compromising long-term performance in vacuum systems. The chemical inertness and robustness of ruthenium contribute to its enhanced durability, making it a preferred choice for demanding applications requiring sustained getter activity.
Lifespan and Regeneration Capabilities
Ruthenium-based getters exhibit longer lifespan due to their high resistance to oxidation and stable chemical properties, maintaining efficiency over extended periods. Barium getters offer superior regeneration capabilities by easily reactivating through heating, allowing restoration of sorption capacity multiple times. Choosing between Ruthenium and Barium depends on application demands for durability versus ease of in-situ regeneration in vacuum and electronic device systems.
Compatibility with Different Systems
Ruthenium excels as a getter material in vacuum systems due to its high chemical stability and excellent sorption properties, making it compatible with complex semiconductor and thin-film deposition processes. Barium, while effective in ultra-high vacuum environments because of its strong gettering capacity for oxygen and nitrogen, can be less suitable in systems sensitive to contamination or where precise control of reactive gases is critical. Compatibility considerations must weigh Ruthenium's inertness and long-term durability against Barium's aggressive reactivity and potential for undesired byproduct formation in various vacuum technologies.
Safety and Toxicity Considerations
Ruthenium as a getter material exhibits lower toxicity and improved chemical stability compared to barium, which is highly reactive and poses significant safety hazards due to its corrosiveness and potential to form toxic compounds. Ruthenium's inertness reduces risks during handling and operation in vacuum systems, minimizing exposure to hazardous substances. In contrast, barium requires stringent safety protocols to prevent chemical burns and respiratory exposure, making ruthenium a safer alternative in getter applications.
Cost and Availability Comparison
Ruthenium and Barium serve as getters in vacuum systems, with ruthenium offering high chemical stability and efficient gas absorption but at significantly higher cost due to its rarity and complex extraction process. Barium is more abundant and economically viable, providing effective gettering properties at a lower price, which makes it preferable for large-scale industrial applications. Availability challenges for ruthenium stem from limited mining locations and geopolitical factors, while barium's widespread natural occurrence ensures more consistent supply.
Choosing the Right Getter: Key Factors
Ruthenium excels as a getter material due to its superior chemical stability and strong affinity for oxygen and hydrogen, enhancing vacuum quality in electronic applications. Barium offers exceptional reactivity and low vapor pressure, making it ideal for rapid gas sorption in vacuum systems but may require careful handling due to its higher chemical activity. Choosing the right getter depends on application-specific factors such as operating temperature, vacuum level, and compatibility with system materials to optimize performance and longevity.
Infographic: Ruthenium vs Barium for Getter
 azmater.com
azmater.com