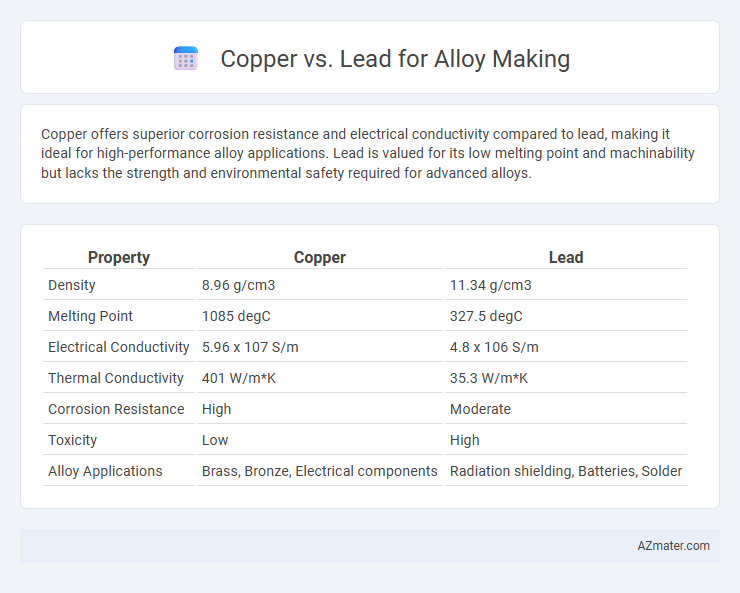
Copper offers superior corrosion resistance and electrical conductivity compared to lead, making it ideal for high-performance alloy applications. Lead is valued for its low melting point and machinability but lacks the strength and environmental safety required for advanced alloys.
Table of Comparison
| Property | Copper | Lead |
|---|---|---|
| Density | 8.96 g/cm3 | 11.34 g/cm3 |
| Melting Point | 1085 degC | 327.5 degC |
| Electrical Conductivity | 5.96 x 107 S/m | 4.8 x 106 S/m |
| Thermal Conductivity | 401 W/m*K | 35.3 W/m*K |
| Corrosion Resistance | High | Moderate |
| Toxicity | Low | High |
| Alloy Applications | Brass, Bronze, Electrical components | Radiation shielding, Batteries, Solder |
Introduction to Alloy Making
Copper and lead are both fundamental metals used in alloy making, distinguished by their contrasting properties and applications. Copper offers excellent electrical conductivity and corrosion resistance, making it ideal for alloys such as bronze and brass, which combine strength and durability. Lead contributes superior density and malleability, often alloyed for applications requiring enhanced soundproofing and radiation shielding.
Overview of Copper and Lead
Copper, a highly conductive and corrosion-resistant metal, is widely used in alloy making to enhance strength and electrical properties, especially in brass and bronze. Lead, known for its high density and malleability, is often added to alloys to improve machinability and resistance to wear and corrosion. Combining copper and lead in alloys leverages copper's durability with lead's lubricating qualities, resulting in materials suited for bearings, plumbing, and electrical components.
Physical Properties Comparison
Copper exhibits higher electrical and thermal conductivity compared to lead, making it ideal for applications requiring efficient energy transfer. Lead is denser and softer, with a melting point significantly lower than copper's 327.5degC, facilitating easier casting and shaping but reducing structural strength. The tensile strength of copper far exceeds lead's, providing enhanced durability in alloy formulations.
Corrosion Resistance: Copper vs Lead
Copper exhibits superior corrosion resistance compared to lead, especially in atmospheric and marine environments, due to its ability to form a stable oxide layer that protects the underlying metal. Lead, while highly resistant to acids and certain chemical environments, tends to corrode more rapidly when exposed to alkaline conditions and lacks the protective oxide film that copper develops. In alloy making, copper's enhanced corrosion resistance makes it the preferred choice for applications requiring durability and long-term exposure to corrosive elements.
Mechanical Strength and Durability
Copper alloys exhibit superior mechanical strength and durability compared to lead-based alloys, making them ideal for high-stress applications. The tensile strength of copper alloys ranges from 200 to 700 MPa, significantly outperforming lead alloys, which typically have tensile strengths below 50 MPa. Copper's corrosion resistance also contributes to its longer lifespan and enhanced durability in harsh environments.
Conductivity Differences
Copper exhibits significantly higher electrical conductivity than lead, making it the preferred choice for applications requiring efficient electrical flow. While lead offers greater density and corrosion resistance, its conductivity is roughly 17% that of copper, which limits its use in electrical alloys. The superior conductivity of copper alloys enhances performance in wiring, electronics, and electromagnets, where minimizing energy loss is critical.
Environmental Impact and Safety
Copper is generally favored over lead in alloy making due to its lower toxicity and reduced environmental impact, as lead exposure can cause severe health issues and contaminate soil and water. Copper alloys offer better recyclability and durability, minimizing waste and environmental degradation. Regulatory restrictions on lead use further promote copper's safer profile in manufacturing applications.
Cost and Availability
Copper is generally more expensive than lead due to its higher demand in electrical and construction industries, making cost a crucial factor in alloy selection. Lead is more abundant and easier to source, which lowers its price and improves availability for alloy production. Industries often balance cost-effectiveness and material properties when choosing between copper and lead alloys.
Common Applications in Alloys
Copper alloys, such as bronze and brass, are widely used in electrical connectors, plumbing fixtures, and marine hardware due to their excellent corrosion resistance, electrical conductivity, and malleability. Lead alloys, commonly combined with tin and antimony, are favored in battery grids, radiation shielding, and soldering applications because of their high density, low melting point, and superior vibration damping properties. The distinct mechanical and chemical characteristics of copper and lead alloys dictate their specialized roles in automotive, aerospace, and construction industries.
Conclusion: Choosing the Right Metal
Selecting copper or lead for alloy making depends on the specific application requirements such as corrosion resistance, electrical conductivity, and toxicity. Copper alloys excel in electrical and thermal conductivity, mechanical strength, and corrosion resistance, making them ideal for electronics and plumbing. Lead alloys offer excellent machinability and radiation shielding but are limited by their toxicity and lower mechanical strength, making copper generally the preferred choice for most modern alloy applications.
Infographic: Copper vs Lead for Alloy Making
 azmater.com
azmater.com