Gallium offers higher thermal stability and conductivity compared to lithium, making it a promising alternative for high-performance batteries. Lithium remains dominant due to its lightweight properties and established energy density in commercial applications.
Table of Comparison
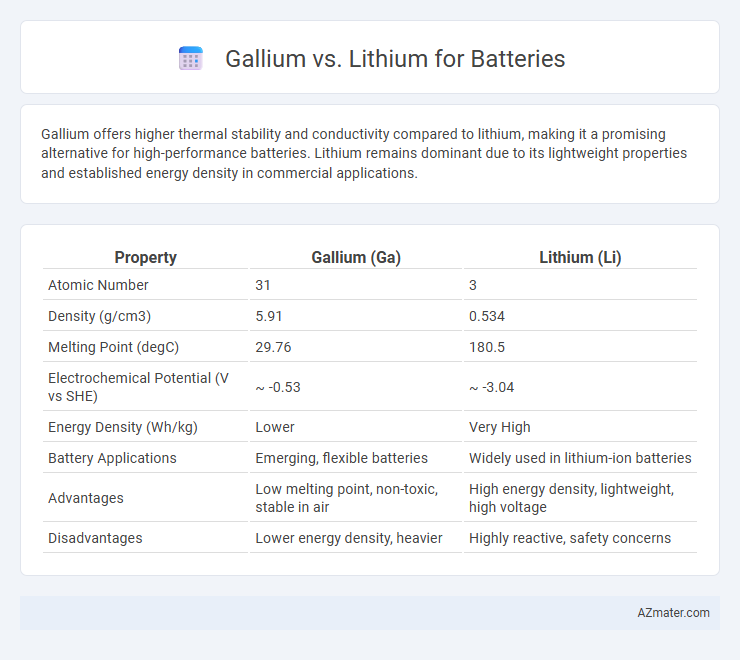
| Property | Gallium (Ga) | Lithium (Li) |
|---|---|---|
| Atomic Number | 31 | 3 |
| Density (g/cm3) | 5.91 | 0.534 |
| Melting Point (degC) | 29.76 | 180.5 |
| Electrochemical Potential (V vs SHE) | ~ -0.53 | ~ -3.04 |
| Energy Density (Wh/kg) | Lower | Very High |
| Battery Applications | Emerging, flexible batteries | Widely used in lithium-ion batteries |
| Advantages | Low melting point, non-toxic, stable in air | High energy density, lightweight, high voltage |
| Disadvantages | Lower energy density, heavier | Highly reactive, safety concerns |
Introduction to Battery Materials: Gallium and Lithium
Gallium and lithium represent two critical materials in advanced battery technology, each influencing energy storage performance through distinct electrochemical properties. Lithium, known for its high electrochemical potential and low atomic weight, remains the dominant element in commercial rechargeable batteries, driving high energy density and efficient charge cycles. Gallium, with its unique ability to form liquid alloys and resist dendrite formation, emerges as a promising alternative or supplement to lithium, potentially enhancing battery safety and cycle life in next-generation energy storage systems.
Chemical Properties: Gallium vs Lithium
Gallium exhibits a low melting point of 29.8degC and remains liquid near room temperature, contrasting with lithium's solid state and melting point of 180.5degC. Chemically, lithium is highly reactive, especially with water, forming lithium hydroxide and releasing hydrogen gas, while gallium is less reactive and forms a stable oxide layer. The difference in electrochemical potential is notable; lithium's standard reduction potential (-3.04 V) is lower than gallium's (-0.56 V), making lithium more efficient for high-energy-density batteries.
Abundance and Sourcing of Gallium and Lithium
Gallium is significantly less abundant in the Earth's crust, with an average concentration of about 18 parts per million compared to lithium's 20 parts per million, but lithium's commercial extraction is more developed due to its concentration in brine deposits and hard rock minerals. Lithium's primary sourcing regions include Australia, Chile, and China, where large-scale mining operations and brine extraction provide a steady supply, while gallium is primarily obtained as a byproduct of aluminum and zinc refining, making its sourcing more dependent on the steel and semiconductor industries. The scalability of lithium extraction methods currently surpasses gallium, although gallium's integration into battery technology is limited by its lower availability and more complex recovery processes.
Efficiency and Energy Density Comparison
Gallium batteries offer higher thermal stability and longer cycle life compared to lithium batteries, enhancing overall efficiency in high-temperature applications. Lithium batteries maintain superior energy density, typically around 150-250 Wh/kg, whereas gallium-based batteries currently achieve lower energy densities, limiting their portability advantages. Efficiency in gallium batteries benefits from reduced dendrite formation, resulting in safer and more consistent energy delivery despite lithium's dominance in energy density metrics.
Safety and Stability: Gallium vs Lithium Batteries
Gallium batteries exhibit superior safety and stability compared to lithium batteries due to gallium's higher melting point and lower tendency to form dendrites, which reduces risks of short circuits and thermal runaway. Lithium batteries, while energy-dense, often face challenges with lithium dendrite growth causing internal short-circuits and potential fires. Gallium's non-reactive nature and stable liquid state at near-room temperatures make it a promising candidate for safer, more thermally stable battery technologies.
Environmental Impact and Sustainability
Gallium batteries offer enhanced recyclability and lower toxicity compared to lithium, reducing environmental pollution and easing battery disposal challenges. Lithium extraction involves significant ecological disruption, including water depletion and habitat damage, which raises sustainability concerns. Gallium's abundance and less harmful mining processes contribute to its potential as a more eco-friendly, sustainable alternative in battery technology.
Cost Analysis: Gallium Batteries vs Lithium Batteries
Gallium batteries generally incur higher material costs due to the rarity and complex extraction process of gallium compared to the more abundant lithium used in lithium-ion batteries. Manufacturing expenses for gallium-based batteries are elevated owing to specialized technology requirements and limited large-scale production facilities, contrasting with the well-established, cost-efficient lithium battery supply chain. Despite higher upfront costs, gallium batteries offer potential savings in longevity and performance efficiency, which may offset initial investment over time.
Technological Challenges and Limitations
Gallium batteries face technological challenges such as limited electrochemical stability and difficulties in achieving high energy density compared to lithium-ion batteries. Lithium technology struggles with issues like dendrite formation, thermal runaway, and finite resource availability, impacting safety and scalability. Both materials require advances in electrode design, electrolyte formulation, and manufacturing processes to overcome current limitations and improve performance for future energy storage applications.
Current Applications and Future Potential
Gallium-based batteries offer promising advantages in high-temperature stability and flexibility, making them suitable for specialized electronics and emerging wearable devices, whereas lithium-ion batteries dominate the market due to their high energy density and extensive infrastructure support. Research into gallium's low toxicity and abundant availability highlights its future potential for safer, environmentally friendly battery technologies. Lithium batteries continue to improve in energy capacity and cost-efficiency, but gallium innovations could lead to next-generation batteries with enhanced durability and sustainable production methods.
Conclusion: Which Material Leads the Future of Batteries?
Gallium and lithium each offer unique advantages in battery technology, with lithium dominating current energy storage due to its high energy density and established infrastructure. Gallium shows promise for safer, more flexible batteries because of its low melting point and non-toxic properties, potentially revolutionizing wearable and flexible electronics. The future of batteries may hinge on integrating gallium's innovative material benefits with lithium's proven performance to meet evolving energy and safety demands.
Infographic: Gallium vs Lithium for Battery
 azmater.com
azmater.com