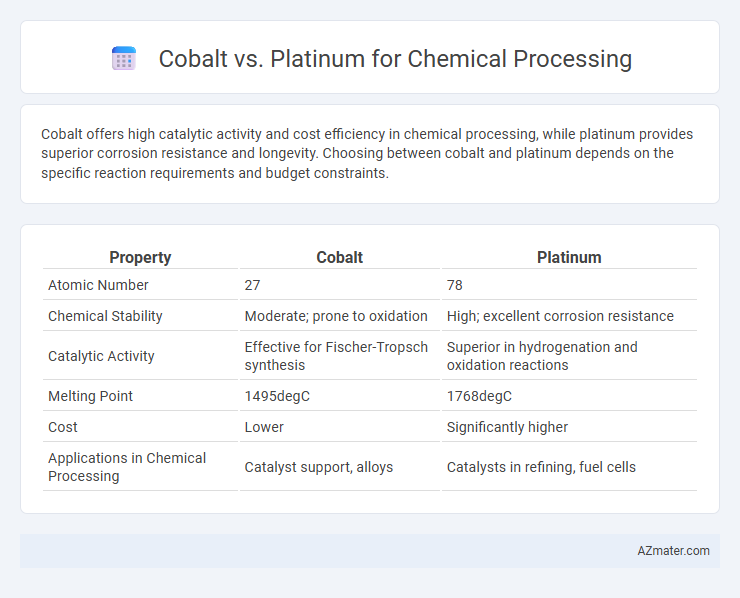
Cobalt offers high catalytic activity and cost efficiency in chemical processing, while platinum provides superior corrosion resistance and longevity. Choosing between cobalt and platinum depends on the specific reaction requirements and budget constraints.
Table of Comparison
| Property | Cobalt | Platinum |
|---|---|---|
| Atomic Number | 27 | 78 |
| Chemical Stability | Moderate; prone to oxidation | High; excellent corrosion resistance |
| Catalytic Activity | Effective for Fischer-Tropsch synthesis | Superior in hydrogenation and oxidation reactions |
| Melting Point | 1495degC | 1768degC |
| Cost | Lower | Significantly higher |
| Applications in Chemical Processing | Catalyst support, alloys | Catalysts in refining, fuel cells |
Introduction to Cobalt and Platinum in Chemical Processing
Cobalt and platinum play critical roles in chemical processing due to their unique catalytic properties. Cobalt is widely used as a catalyst in Fischer-Tropsch synthesis and hydroformylation reactions, offering cost-effective performance in producing synthetic fuels and chemicals. Platinum, known for its exceptional catalytic activity and resistance to corrosion, is essential in catalytic converters, hydrogenation, and fuel cell applications, providing high efficiency and durability in various industrial processes.
Elemental Properties and Structure
Cobalt exhibits a hexagonal close-packed crystal structure at room temperature, transitioning to face-centered cubic at higher temperatures, which influences its magnetic and catalytic properties in chemical processing. Platinum possesses a face-centered cubic lattice, providing exceptional chemical stability and resistance to corrosion, making it highly effective as a catalyst in various industrial reactions. The electronic configuration of cobalt (3d7 4s2) allows for versatile oxidation states, while platinum's (5d9 6s1) stable d-electron arrangement supports superior catalytic activity in hydrogenation and oxidation reactions.
Catalytic Performance: Cobalt vs Platinum
Cobalt catalysts demonstrate high thermal stability and excellent resistance to sulfur poisoning, making them effective for hydroprocessing in chemical reactions. Platinum catalysts, known for superior hydrogenation activity and selectivity, excel in fine chemical synthesis and fuel cell applications. The choice between cobalt and platinum depends on the specific chemical process requirements, balancing cost efficiency with catalytic performance and durability.
Cost Comparison and Economic Viability
Cobalt catalysts generally offer a lower-cost alternative to platinum in chemical processing applications, with cobalt priced significantly less per ounce, making it more economically viable for large-scale or cost-sensitive operations. While platinum exhibits superior catalytic efficiency and durability, the higher upfront cost and price volatility reduce its attractiveness compared to cobalt in budget-constrained environments. Industries prioritize cobalt when balancing catalytic performance with cost-effectiveness, especially in processes like Fischer-Tropsch synthesis and hydroprocessing where cost impact is substantial.
Corrosion Resistance and Durability
Cobalt exhibits superior corrosion resistance and durability in harsh chemical processing environments due to its high resistance to oxidizing and reducing agents, making it ideal for components exposed to aggressive solvents and acids. Platinum, while exceptional for catalytic applications and resistant to corrosion by most chemicals, is softer and less durable under mechanical stress compared to cobalt alloys. In industrial chemical processing, cobalt-based materials are often preferred for their ability to maintain structural integrity and resist wear over prolonged exposure to corrosive substances.
Environmental Impact and Sustainability
Cobalt and platinum both play crucial roles in chemical processing, but their environmental impacts differ significantly; cobalt mining often leads to habitat destruction and toxic waste, raising concerns about sustainable sourcing. Platinum, while rarer and more expensive, offers higher catalytic efficiency and durability, reducing the frequency of replacement and associated environmental footprint. Sustainable chemical processing increasingly favors catalysts that balance performance with responsible resource management, highlighting the importance of recycling and alternative materials in the cobalt-platinum debate.
Industrial Applications and Use Cases
Cobalt catalysts are widely used in Fischer-Tropsch synthesis and hydrocarbon reforming due to their high activity and selectivity, especially in producing synthetic fuels and chemicals. Platinum excels in catalytic converters and hydrogenation reactions within pharmaceutical and fine chemical manufacturing, offering superior stability and resistance to poisoning in harsh industrial environments. Both metals serve crucial roles in chemical processing, with cobalt favored for large-scale hydrocarbon transformations and platinum preferred for precision catalysis and environmental applications.
Availability and Supply Chain Considerations
Cobalt is more abundant and widely available than platinum, primarily mined in the Democratic Republic of Congo, which poses geopolitical risks and potential supply chain disruptions. Platinum, rarer and predominantly sourced from South Africa and Russia, faces limited mine outputs and higher extraction costs, impacting its consistent availability for chemical processing applications. Supply chain challenges for both metals include fluctuating demand in industrial sectors and the need for sustainable, ethically sourced materials to ensure long-term accessibility.
Safety and Handling Requirements
Cobalt requires strict handling protocols due to its toxic dust and possible carcinogenic effects, necessitating the use of personal protective equipment (PPE) and adequate ventilation in chemical processing environments. Platinum, while generally considered less toxic, demands precautions against exposure to soluble salts, which can cause skin and respiratory irritation, and requires controlled handling to prevent costly contamination or loss. Both metals necessitate compliance with occupational safety standards such as OSHA and proper waste disposal procedures to mitigate environmental and health risks.
Future Trends and Research Directions
Cobalt-based catalysts are gaining momentum in chemical processing due to their cost-effectiveness and robust activity in Fischer-Tropsch synthesis, making them a promising alternative to platinum in hydrocarbon conversion. Research increasingly targets enhancing cobalt catalyst stability through nanoscale engineering and alloying to improve resistance against sintering and poisoning, addressing key limitations faced by platinum catalysts. Emerging trends emphasize sustainable catalyst design, integrating cobalt with earth-abundant materials to optimize performance and reduce dependency on scarce platinum resources in future industrial applications.
Infographic: Cobalt vs Platinum for Chemical processing
 azmater.com
azmater.com