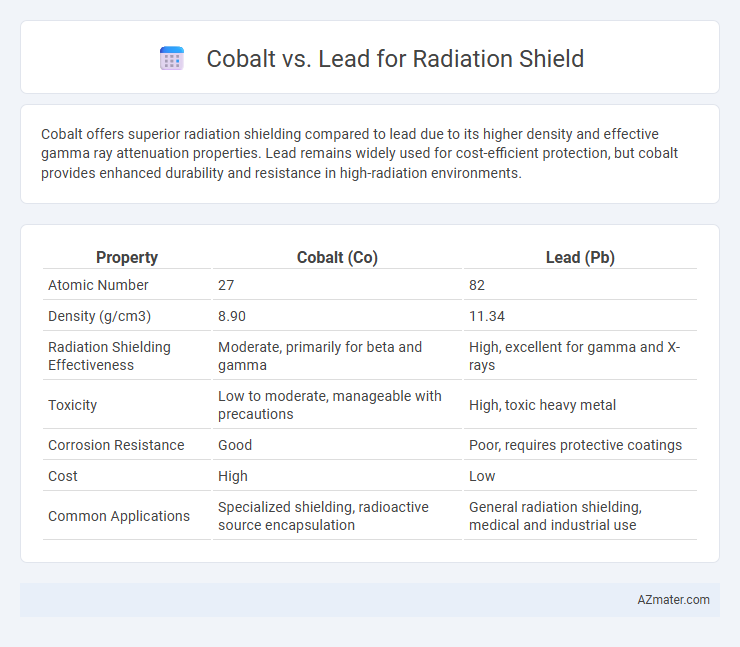
Cobalt offers superior radiation shielding compared to lead due to its higher density and effective gamma ray attenuation properties. Lead remains widely used for cost-efficient protection, but cobalt provides enhanced durability and resistance in high-radiation environments.
Table of Comparison
| Property | Cobalt (Co) | Lead (Pb) |
|---|---|---|
| Atomic Number | 27 | 82 |
| Density (g/cm3) | 8.90 | 11.34 |
| Radiation Shielding Effectiveness | Moderate, primarily for beta and gamma | High, excellent for gamma and X-rays |
| Toxicity | Low to moderate, manageable with precautions | High, toxic heavy metal |
| Corrosion Resistance | Good | Poor, requires protective coatings |
| Cost | High | Low |
| Common Applications | Specialized shielding, radioactive source encapsulation | General radiation shielding, medical and industrial use |
Introduction to Radiation Shielding
Radiation shielding involves materials that absorb or block ionizing radiation to protect humans and sensitive equipment. Lead is a traditional choice due to its high density and atomic number, effectively attenuating gamma rays and X-rays. Cobalt, although less dense, is primarily used as a radioactive source rather than a shield, making lead the preferred material for shielding applications.
Overview of Cobalt and Lead as Shielding Materials
Cobalt and lead are both used as radiation shielding materials, but lead is far more common due to its high density (11.34 g/cm3) and atomic number (82), which provide superior gamma radiation attenuation. Cobalt, with a lower density (8.9 g/cm3) and atomic number (27), is less effective in blocking radiation and is primarily used in specialized applications such as radioactive sources rather than as bulk shielding. Lead's toxicity and ease of fabrication continue to make it the preferred material for protective barriers in medical and industrial radiology environments.
Physical and Chemical Properties Comparison
Cobalt offers superior radiation shielding compared to lead due to its higher atomic number (27 vs. 82), density (8.9 g/cm3 vs. 11.34 g/cm3), and greater resistance to corrosion, enhancing durability in harsh environments. Lead's high density and atomic number make it effective for blocking gamma rays and X-rays, but its toxicity and softness limit its application in certain settings. Cobalt's enhanced mechanical strength and melting point (1495degC vs. 327.5degC for lead) provide advantages in long-term structural radiation shielding solutions.
Effectiveness in Blocking Different Radiation Types
Cobalt and lead differ significantly in their effectiveness at blocking various types of radiation due to their atomic properties and density. Lead, with a high density of 11.34 g/cm3 and an atomic number of 82, is highly effective at attenuating gamma rays and X-rays through photoelectric absorption and Compton scattering. Cobalt, with an atomic number of 27 and lower density around 8.9 g/cm3, is less effective against high-energy photons but can be useful in neutron radiation shielding when alloyed or combined with other materials.
Density and Atomic Number: Shielding Implications
Cobalt, with an atomic number of 27 and a density of 8.90 g/cm3, offers moderate radiation shielding capabilities, primarily for gamma and neutron radiation. Lead, possessing a higher atomic number of 82 and a greater density of 11.34 g/cm3, provides superior attenuation of ionizing radiation, making it more effective in blocking X-rays and gamma rays. The higher atomic number and density of lead result in greater photon interaction probability, enhancing its shielding performance compared to cobalt.
Durability and Longevity in Practical Applications
Cobalt offers superior durability compared to lead in radiation shielding due to its higher melting point of 1495degC and excellent corrosion resistance, making it ideal for long-term use in harsh environments. Lead, although dense with a density of 11.34 g/cm3, is prone to oxidation and deformation over time, which can compromise its shielding effectiveness. Practical applications favor cobalt for extended service life, especially in high-temperature and chemically aggressive conditions, while lead remains cost-effective but less durable for static or short-term shielding needs.
Safety and Toxicity Concerns
Cobalt and lead have distinct safety and toxicity profiles when used as radiation shields; lead is highly effective but poses significant health hazards due to its neurotoxicity and potential for chronic lead poisoning through inhalation or ingestion of lead dust or fumes. Cobalt, particularly cobalt-60, is radioactive itself, requiring strict handling protocols to avoid radiation exposure, but it does not present the same chemical toxicity risks as lead. Regulatory guidelines prioritize minimizing exposure to both materials, emphasizing containment, ventilation, and protective equipment to reduce risks associated with lead toxicity and cobalt radioactivity during shield use.
Cost and Availability Analysis
Cobalt is significantly more expensive than lead, with prices driven by its use in medical and industrial applications, making lead the more cost-effective choice for large-scale radiation shielding projects. Lead is abundantly available as a byproduct of mining and recycling, ensuring steady supply and lower costs, whereas cobalt's limited global reserves and geopolitical factors restrict its availability and increase price volatility. The affordability and widespread availability of lead make it the preferred material for most conventional radiation shielding needs despite cobalt's higher density and superior shielding efficiency.
Typical Use Cases in Medicine and Industry
Cobalt, primarily in the form of cobalt-60, is widely used in medical radiation therapy for cancer treatment due to its consistent gamma-ray emission, enabling precise tumor targeting and sterilization applications in both healthcare and industrial radiography. Lead's high density makes it the preferred material for protective shielding in X-ray rooms, nuclear facilities, and radiology departments, effectively blocking scattered and primary radiation to ensure safety for patients and operators. Industrially, cobalt sources facilitate non-destructive testing and material analysis, while lead remains essential for portable shields, container linings, and structural barriers in radiation environments.
Conclusion: Choosing the Right Material
Selecting between cobalt and lead for radiation shielding depends on balancing factors like density, cost, and toxicity. Lead offers superior radiation attenuation due to its high atomic number and density, making it a cost-effective choice for many applications despite its toxicity. Cobalt, while less dense and more expensive, provides better structural strength and corrosion resistance, benefiting specialized environments requiring durability alongside radiation protection.
Infographic: Cobalt vs Lead for Radiation Shield
 azmater.com
azmater.com