Bismuth provides low toxicity and excellent machinability in alloys, while gold offers superior corrosion resistance and electrical conductivity. Alloys with bismuth are ideal for environmentally friendly applications, whereas gold alloys excel in precision electronics and luxury products.
Table of Comparison
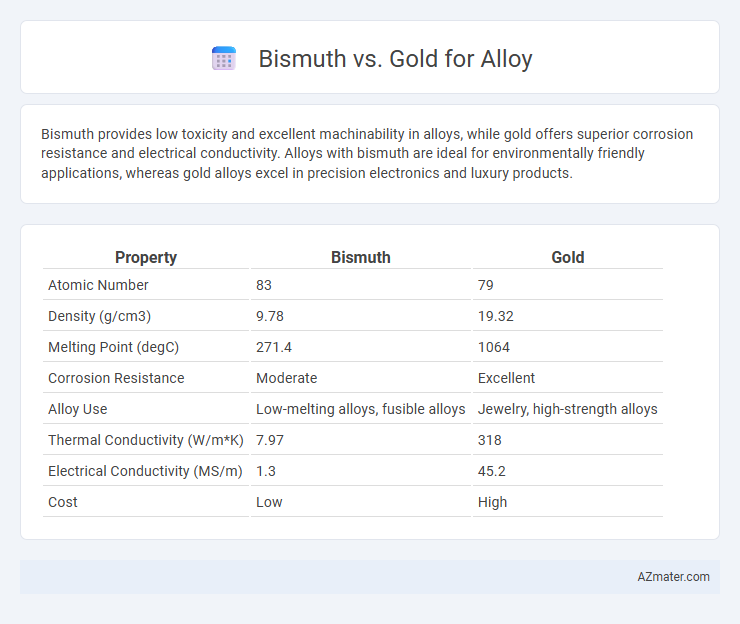
| Property | Bismuth | Gold |
|---|---|---|
| Atomic Number | 83 | 79 |
| Density (g/cm3) | 9.78 | 19.32 |
| Melting Point (degC) | 271.4 | 1064 |
| Corrosion Resistance | Moderate | Excellent |
| Alloy Use | Low-melting alloys, fusible alloys | Jewelry, high-strength alloys |
| Thermal Conductivity (W/m*K) | 7.97 | 318 |
| Electrical Conductivity (MS/m) | 1.3 | 45.2 |
| Cost | Low | High |
Introduction to Bismuth and Gold as Alloy Materials
Bismuth and gold alloys exhibit distinct material properties vital for various industrial applications, with bismuth known for its low toxicity, high density, and excellent machinability, making it an eco-friendly alternative in metal alloys. Gold alloys combine gold's exceptional corrosion resistance, high ductility, and aesthetic appeal, often used in jewelry and electronics where durability and conductivity are essential. Understanding the fundamental characteristics of bismuth's brittleness and gold's malleability aids in selecting the appropriate alloy composition for specialized uses such as safe metal casting, thermal conductivity, and biocompatible medical devices.
Physical Properties Comparison: Bismuth vs Gold
Bismuth exhibits a density of 9.78 g/cm3 and a melting point of 271.5degC, significantly lower than gold's density of 19.32 g/cm3 and melting point of 1064degC, influencing alloy weight and thermal stability. Bismuth is brittle with low ductility, contrasting gold's exceptional malleability and ductility, which affect the mechanical strength and flexibility of alloys. Thermal and electrical conductivities differ substantially, with gold having high conductivity, making gold alloys preferable for electronic applications, while bismuth alloys are often used for their low thermal conductivity and unique expansion properties.
Melting Points and Alloy Formation
Bismuth has a melting point of 271.5degC, significantly lower than gold's melting point of 1064degC, which allows for easy alloying and melting in low-temperature applications. When combined, bismuth and gold alloys form with enhanced machinability and unique physical properties, often used in specialized jewelry and electronics. The difference in melting points enables precise control during alloy formation, resulting in materials with tailored hardness and corrosion resistance.
Electrical and Thermal Conductivity Differences
Bismuth exhibits significantly lower electrical conductivity compared to gold, making gold a preferred choice for high-conductivity alloy applications in electronics. Thermal conductivity further differentiates these metals, with gold providing superior heat dissipation capabilities essential for thermal management in electronic components. Incorporating bismuth can reduce overall alloy conductivity but improve machinability and reduce cost, while gold enhances both electrical and thermal performance.
Corrosion Resistance in Alloys
Bismuth alloys exhibit superior corrosion resistance compared to gold-based alloys, especially in acidic and marine environments. Gold alloys, while highly resistant to oxidation and tarnish, are more prone to localized corrosion in the presence of halides. The unique passivation properties of bismuth contribute to enhanced durability and longer service life in corrosive applications.
Popular Bismuth-Based Alloy Applications
Bismuth-based alloys are widely favored for their low melting points, non-toxicity, and excellent machinability, making them ideal for applications such as fire detection systems, metal casting, and medical devices. In contrast, gold alloys, while highly corrosion-resistant and electrically conductive, are typically reserved for high-end electronics, jewelry, and specialized aerospace components due to their high cost. The unique properties of bismuth alloys, including their environmentally friendly nature and ability to form eutectic mixtures with lead-free characteristics, are driving their popularity in modern industrial and safety applications.
Gold Alloys in Industry and Jewelry
Gold alloys dominate the jewelry industry due to their superior luster, durability, and resistance to tarnish, making them ideal for crafting intricate designs. Incorporating metals like copper, silver, and palladium enhances gold's hardness without compromising its malleability, essential for both decorative and industrial applications. Bismuth, while environmentally friendly and non-toxic, is less favored in gold alloys because it can reduce ductility and affect the color consistency, limiting its use primarily to niche industrial sectors rather than mainstream jewelry production.
Cost and Availability of Bismuth vs Gold
Bismuth offers a significantly lower cost compared to gold, making it an economical choice for alloy production where budget constraints are critical. The abundant availability of bismuth in the earth's crust ensures a steady supply, contrasting with gold's limited reserves and higher extraction expenses. These factors contribute to bismuth's growing adoption in alloys for industrial applications where cost-efficiency and material accessibility are prioritized over luxury value.
Environmental and Health Considerations
Bismuth alloys are environmentally favorable due to their non-toxic and biodegradable properties, posing minimal health risks during processing and disposal, unlike gold alloys that can involve toxic refining processes with environmental pollutants such as mercury and cyanide. Bismuth's low toxicity makes it suitable for medical and cosmetic applications, reducing occupational hazards commonly associated with gold alloy mining and smelting. The sustainable sourcing of bismuth also contributes to lower carbon emissions and less ecological disruption compared to the extensive gold mining industry.
Choosing the Right Metal for Alloying Purposes
Bismuth offers lower density and excellent machinability compared to gold, making it ideal for alloys requiring non-toxicity and environmental safety. Gold provides superior corrosion resistance and electrical conductivity, essential for high-performance and decorative alloys. Selecting between bismuth and gold depends on the alloy's required mechanical properties, cost efficiency, and application context such as electronics or jewelry.
Infographic: Bismuth vs Gold for Alloy
 azmater.com
azmater.com