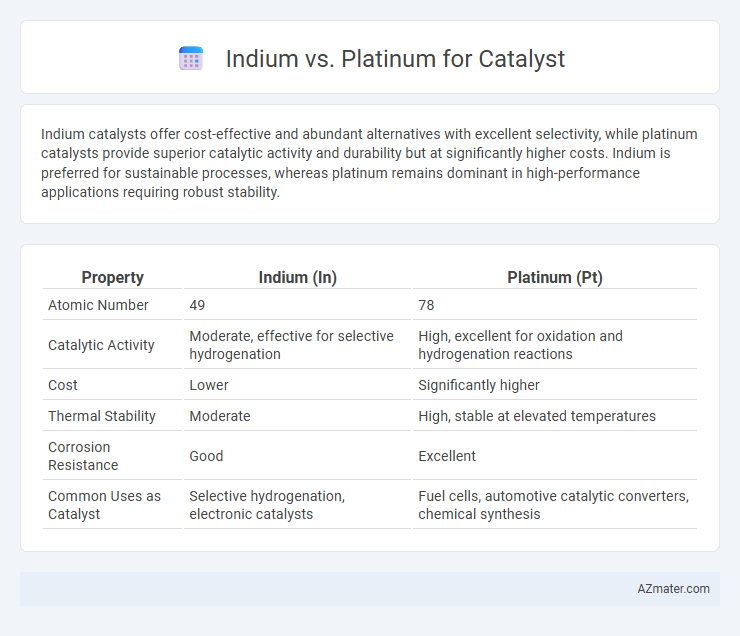
Indium catalysts offer cost-effective and abundant alternatives with excellent selectivity, while platinum catalysts provide superior catalytic activity and durability but at significantly higher costs. Indium is preferred for sustainable processes, whereas platinum remains dominant in high-performance applications requiring robust stability.
Table of Comparison
| Property | Indium (In) | Platinum (Pt) |
|---|---|---|
| Atomic Number | 49 | 78 |
| Catalytic Activity | Moderate, effective for selective hydrogenation | High, excellent for oxidation and hydrogenation reactions |
| Cost | Lower | Significantly higher |
| Thermal Stability | Moderate | High, stable at elevated temperatures |
| Corrosion Resistance | Good | Excellent |
| Common Uses as Catalyst | Selective hydrogenation, electronic catalysts | Fuel cells, automotive catalytic converters, chemical synthesis |
Introduction to Indium and Platinum as Catalysts
Indium and platinum serve distinct roles as catalysts, with platinum widely recognized for its superior catalytic efficiency in processes like hydrogenation and fuel cells due to its high stability and excellent electron transfer properties. Indium, though less common, is valued in catalysis for its unique ability to promote selective reactions, particularly in organic synthesis and CO2 reduction, benefiting from its lower cost and abundance compared to platinum. Both metals enable pivotal chemical transformations, yet platinum's established performance contrasts with indium's emerging potential in sustainable catalytic applications.
Chemical Properties and Composition
Indium, a post-transition metal with atomic number 49, exhibits lower electronegativity and a softer electron configuration, making it less effective in catalytic oxidation reactions compared to platinum. Platinum, a transition metal with atomic number 78, has a dense d-electron shell that facilitates strong adsorption and activation of reactants, enhancing catalytic efficiency in processes like hydrogenation and fuel cells. The chemical composition of platinum alloys often incorporates elements to improve durability and catalytic selectivity, whereas indium catalysts tend to focus on applications requiring lower temperature stability and unique electronic interactions.
Activity and Selectivity in Catalytic Reactions
Indium exhibits high selectivity in catalytic reactions such as hydrogenation and CO2 reduction, favoring the formation of specific products like formic acid with minimal side reactions. Platinum demonstrates superior catalytic activity due to its ability to facilitate various reaction pathways efficiently, but often produces a broader range of products, reducing selectivity. The choice between indium and platinum catalysts depends on the desired balance between high activity and product specificity in applications like fuel cells and organic synthesis.
Cost and Economic Considerations
Indium catalysts generally cost less than platinum, making them economically attractive for large-scale applications where budget constraints are critical. Platinum's high market price and susceptibility to supply fluctuations significantly increase overall catalyst expenses, despite its superior catalytic efficiency. Evaluating long-term operational costs and potential catalyst poisoning is essential when considering cost-effectiveness between indium and platinum catalysts.
Environmental Impact and Sustainability
Indium exhibits lower environmental toxicity compared to platinum, making it a more sustainable option for catalytic applications in green chemistry. Platinum's extraction and refining processes are energy-intensive and generate significant greenhouse gas emissions, whereas indium can often be sourced as a byproduct from zinc mining, reducing its ecological footprint. Despite platinum's higher catalytic efficiency, indium's abundance and lower environmental impact position it as a promising alternative for sustainable catalysis.
Durability and Longevity in Industrial Use
Indium catalysts exhibit moderate durability with a tendency to oxidize under high-temperature industrial conditions, limiting their longevity compared to platinum. Platinum demonstrates superior resistance to corrosion and thermal degradation, maintaining catalytic activity over extended operational periods in harsh environments. Industrial applications favor platinum for long-term use due to its enhanced durability and consistent performance under rigorous processing conditions.
Applications in Key Industries
Indium serves as an efficient catalyst primarily in organic synthesis and electronics manufacturing due to its low toxicity and high selectivity, particularly in producing semiconductors and indium tin oxide for touchscreens and solar cells. Platinum's superior catalytic properties are critical in automotive catalytic converters, fuel cells, and chemical industries, enabling efficient hydrogen production and exhaust gas treatment. Both metals are essential in green technology, with platinum leading in sustainable energy applications and indium advancing smart device innovations.
Performance in Hydrogenation Reactions
Indium exhibits selective hydrogenation performance with high activity toward reducing carbonyl compounds while minimizing over-hydrogenation, making it ideal for fine chemical synthesis. Platinum demonstrates superior overall catalytic hydrogenation efficiency with robust activity across a broad range of substrates, including alkenes and aromatic compounds, but can lead to less selective outcomes. The choice between indium and platinum catalysts depends on the desired balance between selectivity and hydrogenation rate in targeted hydrogenation reactions.
Recent Advances in Catalyst Technology
Recent advances in catalyst technology highlight indium's emerging role as a cost-effective and efficient alternative to platinum in various catalytic processes, particularly in CO2 reduction and hydrogenation reactions. Indium catalysts demonstrate enhanced selectivity and stability under mild reaction conditions, driven by innovations in nanostructuring and alloying techniques. Platinum remains a benchmark for catalytic activity and durability, especially in fuel cells, but rising costs and scarcity have accelerated research towards indium-based catalysts for scalable and sustainable applications.
Future Prospects for Indium and Platinum Catalysts
Indium catalysts exhibit promising future prospects due to their abundant availability, low toxicity, and excellent selectivity in CO2 reduction processes, making them ideal for sustainable energy applications. Platinum catalysts remain highly valued for their exceptional catalytic activity and durability in fuel cell technologies, though their high cost and scarcity drive research towards cost-effective alternatives. Advances in nanostructuring and alloy development aim to enhance the performance and reduce the reliance on platinum, while indium-based catalysts continue to gain traction in green chemical synthesis and environmental remediation.
Infographic: Indium vs Platinum for Catalyst
 azmater.com
azmater.com