Bismuth alloys offer superior corrosion resistance and lower toxicity compared to silver alloys, which excel in electrical conductivity and thermal performance. Selecting between bismuth and silver alloys depends on the application's priority for environmental safety or conductive efficiency.
Table of Comparison
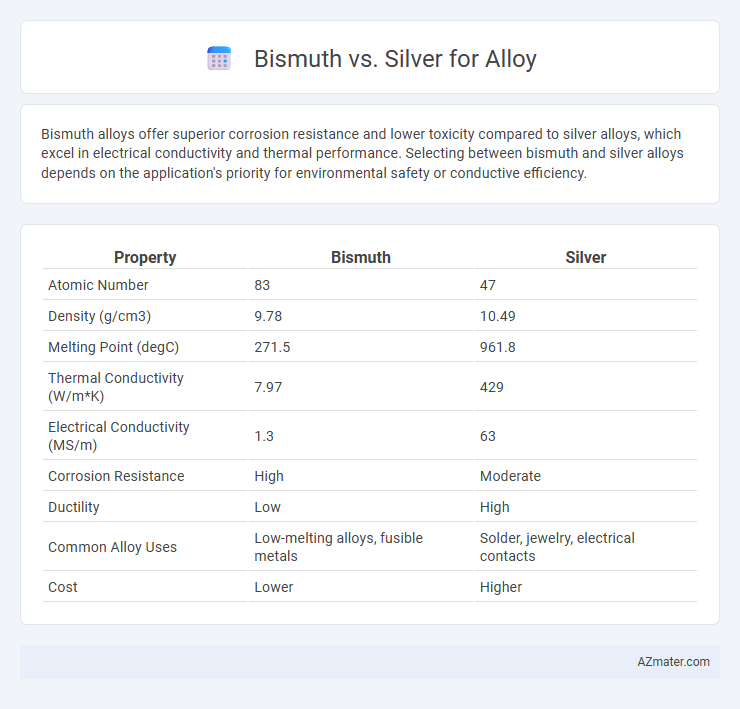
| Property | Bismuth | Silver |
|---|---|---|
| Atomic Number | 83 | 47 |
| Density (g/cm3) | 9.78 | 10.49 |
| Melting Point (degC) | 271.5 | 961.8 |
| Thermal Conductivity (W/m*K) | 7.97 | 429 |
| Electrical Conductivity (MS/m) | 1.3 | 63 |
| Corrosion Resistance | High | Moderate |
| Ductility | Low | High |
| Common Alloy Uses | Low-melting alloys, fusible metals | Solder, jewelry, electrical contacts |
| Cost | Lower | Higher |
Introduction to Bismuth and Silver Alloys
Bismuth and silver alloys are valued for distinct industrial and metallurgical applications due to their unique physical and chemical properties. Bismuth alloys are characterized by their low melting points, non-toxicity, and expansion upon solidification, making them ideal for casting, fire safety devices, and environmentally friendly applications. Silver alloys, known for their high electrical and thermal conductivity, corrosion resistance, and strength, are commonly used in electronics, jewelry, and medical instruments.
Physical Properties Comparison
Bismuth exhibits a lower density of 9.78 g/cm3 compared to silver's 10.49 g/cm3, making bismuth alloys lighter with significant thermal expansion properties. Silver displays superior electrical and thermal conductivity, approximately 63 MS/m and 429 W/m*K respectively, whereas bismuth's conductivity values are much lower, around 0.77 MS/m and 7.97 W/m*K. The melting point of bismuth (271.5degC) is considerably lower than silver's 961.8degC, affecting the casting and processing temperatures in alloy applications.
Chemical Reactivity and Stability
Bismuth exhibits lower chemical reactivity and greater stability compared to silver in alloy applications due to its resistance to oxidation and corrosion. Silver, while highly conductive and ductile, tends to tarnish quickly when exposed to air and sulfur compounds, reducing its long-term stability in alloys. The chemical inertness of bismuth alloys makes them preferable for environments requiring enhanced corrosion resistance and minimal reactivity.
Melting Point Differences
Bismuth has a significantly lower melting point of 271.5degC compared to silver's 961.8degC, which affects their alloying characteristics and processing temperatures. Alloys containing bismuth melt at much lower temperatures, making them ideal for low-temperature soldering and fusible links, while silver alloys require much higher heat, suitable for applications needing greater thermal stability. This melting point difference directly impacts the thermal and mechanical properties of the resulting alloys, influencing their industrial uses and manufacturability.
Alloy Strength and Durability
Bismuth alloys often exhibit lower strength but enhanced machinability and corrosion resistance compared to silver alloys, making them suitable for applications requiring non-toxic and environmentally friendly materials. Silver alloys provide superior tensile strength, hardness, and durability, with excellent thermal and electrical conductivity, which makes them ideal for high-performance and long-lasting components. The choice between bismuth and silver alloys depends on the balance between mechanical strength and specific use-case requirements such as conductivity or environmental safety.
Electrical and Thermal Conductivity
Silver exhibits superior electrical conductivity at 63 x 10^6 S/m and thermal conductivity around 429 W/m*K, making it ideal for applications requiring efficient electrical flow and heat dissipation. Bismuth, with significantly lower electrical conductivity of approximately 1.3 x 10^6 S/m and thermal conductivity near 7.97 W/m*K, is less efficient but adds unique properties like high electronegativity and density when alloyed. Alloys combining bismuth and silver balance conductivity with enhanced physical characteristics such as improved machinability and thermal stability in niche electronic components.
Environmental and Health Considerations
Bismuth alloys offer significant environmental advantages over silver alloys due to bismuth's non-toxic, biodegradable properties, reducing heavy metal pollution and health risks during manufacturing and disposal. Silver, while biocompatible, poses higher environmental concerns given its potential bioaccumulation and extraction-related ecological impacts. Choosing bismuth-based alloys supports safer, sustainable materials in industries prioritizing reduced toxicity and environmental footprint.
Cost and Market Availability
Bismuth is significantly cheaper than silver, making it a cost-effective choice for alloy production, especially in large-scale industrial applications. While silver's price remains high due to its demand in jewelry, electronics, and investment, bismuth's abundance and lower mining costs ensure better market availability. Manufacturers often prefer bismuth alloys for applications requiring non-toxic and low-melting-point properties without the premium cost associated with silver.
Industrial and Commercial Applications
Bismuth alloys offer non-toxic, low-melting-point properties ideal for fire safety devices, medical equipment, and environmentally friendly solders, contrasting with silver alloys, which provide superior electrical conductivity, corrosion resistance, and mechanical strength favored in electronics, jewelry, and high-performance industrial components. Industrial applications utilize bismuth alloys in automatic fire sprinklers and precision fuses, while silver alloys dominate in electrical contacts, photovoltaic cells, and high-quality conductive materials. Commercially, bismuth's cost-effectiveness and eco-friendly nature appeal to sustainable manufacturing sectors, whereas silver's premium status supports luxury products and advanced technology markets.
Choosing the Right Alloy: Bismuth vs Silver
Bismuth and silver serve distinct roles in alloy formulations, with bismuth offering low toxicity and excellent machinability, making it ideal for environmentally safe applications and replacements for lead-containing alloys. Silver alloys provide superior electrical conductivity and corrosion resistance, essential for high-performance electronics and jewelry. Selecting the right alloy depends on balancing factors such as thermal conductivity, cost, mechanical strength, and environmental impact tailored to the specific application requirements.
Infographic: Bismuth vs Silver for Alloy
 azmater.com
azmater.com