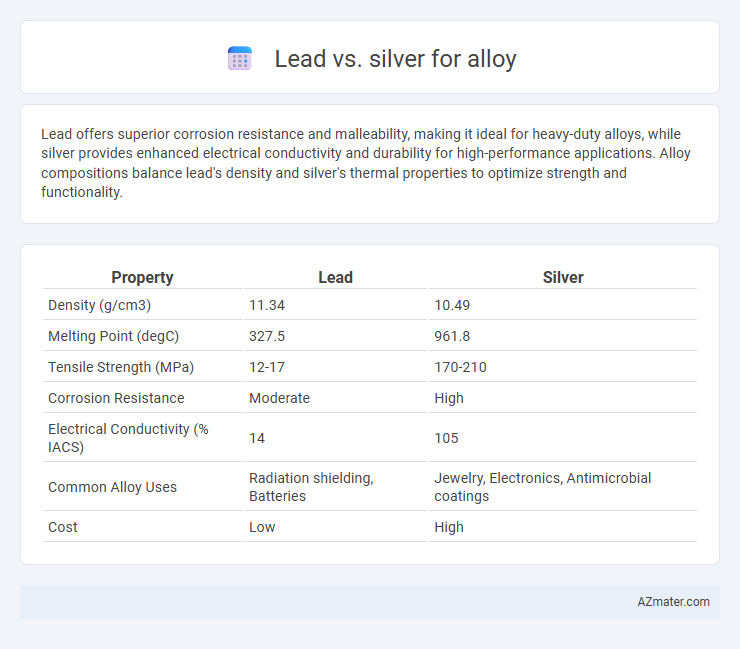
Lead offers superior corrosion resistance and malleability, making it ideal for heavy-duty alloys, while silver provides enhanced electrical conductivity and durability for high-performance applications. Alloy compositions balance lead's density and silver's thermal properties to optimize strength and functionality.
Table of Comparison
| Property | Lead | Silver |
|---|---|---|
| Density (g/cm3) | 11.34 | 10.49 |
| Melting Point (degC) | 327.5 | 961.8 |
| Tensile Strength (MPa) | 12-17 | 170-210 |
| Corrosion Resistance | Moderate | High |
| Electrical Conductivity (% IACS) | 14 | 105 |
| Common Alloy Uses | Radiation shielding, Batteries | Jewelry, Electronics, Antimicrobial coatings |
| Cost | Low | High |
Introduction to Lead and Silver Alloys
Lead alloys are primarily valued for their high density, corrosion resistance, and low melting point, making them ideal in applications like batteries, radiation shielding, and soldering. Silver alloys exhibit excellent electrical and thermal conductivity, along with superior strength and tarnish resistance, which are crucial in electronics, jewelry, and coinage. Both metals enhance base material properties, but lead alloys emphasize durability and protection, whereas silver alloys prioritize conductivity and aesthetic appeal.
Chemical Composition: Lead vs Silver
Lead and silver differ significantly in chemical composition, with lead being a heavy metal characterized by the symbol Pb and atomic number 82, and silver a precious metal denoted by Ag with atomic number 47. Lead's chemical properties include high density, low melting point (327.5degC), and poor electrical conductivity, while silver exhibits superior electrical conductivity, higher melting point (961.8degC), and resistance to oxidation. In alloy formations, lead contributes to malleability and corrosion resistance, whereas silver enhances conductivity and aesthetic appeal, making their chemical differences crucial in selecting materials for specific industrial applications.
Physical Properties Comparison
Lead exhibits higher density (11.34 g/cm3) compared to silver (10.49 g/cm3), resulting in heavier alloys with increased mass per unit volume. Silver outperforms lead in thermal conductivity, with approximately 429 W/m*K, enhancing heat dissipation in alloys, whereas lead's thermal conductivity is much lower at around 35 W/m*K. In terms of hardness, silver alloys typically offer greater strength and durability (Mohs hardness ~2.7) than lead alloys (Mohs hardness ~1.5), influencing wear resistance and structural applications.
Melting Points and Processing
Lead alloys have a melting point typically around 327.5degC, which is significantly lower than silver alloys that melt near 961.8degC, enabling easier casting and molding processes for lead. The lower melting point of lead alloys reduces energy consumption during manufacturing but limits their use in high-temperature applications where silver alloys are preferred for their superior thermal stability. Silver alloys require more precise temperature control and specialized equipment during processing due to their higher melting points, but offer enhanced durability and corrosion resistance in finished products.
Strength and Durability Aspects
Lead alloys exhibit lower strength and durability compared to silver alloys, making them less suitable for applications requiring high mechanical performance. Silver alloys offer superior tensile strength and enhanced resistance to corrosion and wear, contributing to longer service life in demanding environments. The increased hardness of silver-based alloys also provides better structural integrity under stress than lead-based counterparts.
Corrosion Resistance and Longevity
Silver alloys exhibit superior corrosion resistance compared to lead alloys, making them ideal for applications exposed to harsh environments or moisture. Lead alloys tend to corrode more rapidly due to their higher susceptibility to oxidation and acid attack, which reduces their longevity. The enhanced durability of silver alloys contributes to longer-lasting components in industries such as electronics, jewelry, and plumbing.
Common Industrial Applications
Lead alloys are widely used in batteries, radiation shielding, and soldering applications due to lead's excellent density, corrosion resistance, and malleability. Silver alloys find extensive use in electrical contacts, jewelry, and photographic materials because of silver's superior conductivity, antimicrobial properties, and aesthetic appeal. Both metals serve distinct industrial roles where lead provides durability and weight, while silver offers high electrical performance and corrosion resistance.
Environmental and Health Considerations
Lead-based alloys pose significant environmental and health risks due to lead's toxicity, which can cause neurological damage and contamination of soil and water. Silver alloys offer a safer alternative, exhibiting lower toxicity and reduced environmental impact while maintaining desirable metal properties. Regulatory restrictions on lead use in many industries drive the preference for silver alloys in applications that prioritize human health and ecological safety.
Cost and Availability Analysis
Lead alloys generally offer lower material costs compared to silver alloys due to lead's abundant availability and lower market price, making it a cost-effective option for large-scale industrial applications. Silver alloys, while more expensive, provide enhanced durability and corrosion resistance, which can reduce long-term maintenance expenses despite the higher initial investment. The availability of lead remains stable globally, whereas silver supply is more volatile and influenced by mining production and market demand fluctuations.
Choosing the Right Alloy: Lead or Silver
Choosing the right alloy between lead and silver depends on the specific application requirements such as durability, corrosion resistance, and conductivity. Lead alloys excel in shielding against radiation and provide superior machinability, while silver alloys offer excellent electrical conductivity and antimicrobial properties. Evaluating factors like mechanical strength, thermal conductivity, and cost-effectiveness ensures optimal alloy selection for industrial or commercial purposes.
Infographic: Lead vs Silver for Alloy
 azmater.com
azmater.com