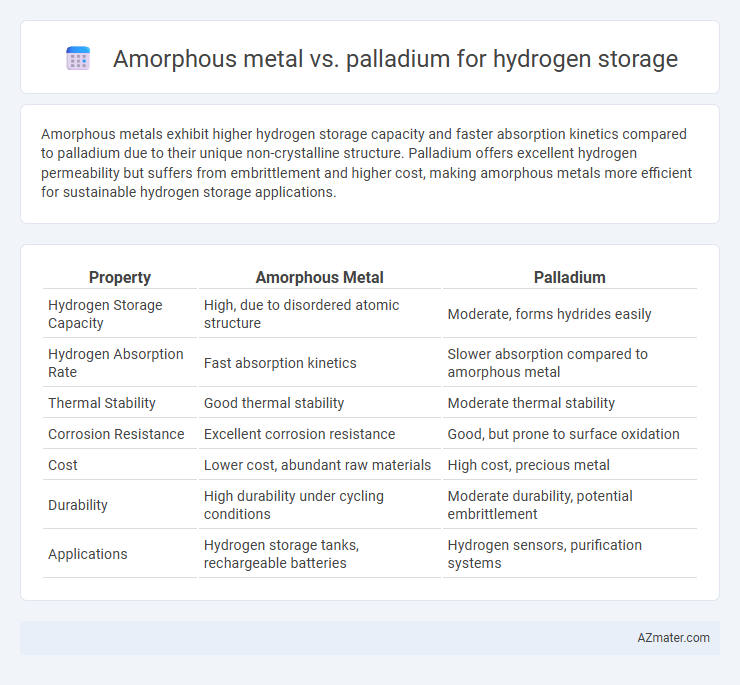
Amorphous metals exhibit higher hydrogen storage capacity and faster absorption kinetics compared to palladium due to their unique non-crystalline structure. Palladium offers excellent hydrogen permeability but suffers from embrittlement and higher cost, making amorphous metals more efficient for sustainable hydrogen storage applications.
Table of Comparison
| Property | Amorphous Metal | Palladium |
|---|---|---|
| Hydrogen Storage Capacity | High, due to disordered atomic structure | Moderate, forms hydrides easily |
| Hydrogen Absorption Rate | Fast absorption kinetics | Slower absorption compared to amorphous metal |
| Thermal Stability | Good thermal stability | Moderate thermal stability |
| Corrosion Resistance | Excellent corrosion resistance | Good, but prone to surface oxidation |
| Cost | Lower cost, abundant raw materials | High cost, precious metal |
| Durability | High durability under cycling conditions | Moderate durability, potential embrittlement |
| Applications | Hydrogen storage tanks, rechargeable batteries | Hydrogen sensors, purification systems |
Introduction to Hydrogen Storage Materials
Hydrogen storage materials such as amorphous metals and palladium play critical roles in advancing clean energy technologies. Amorphous metals exhibit disordered atomic structures that enhance hydrogen absorption kinetics and increase storage capacity due to the absence of grain boundaries. Palladium, renowned for its exceptional hydrogen solubility and reversible absorption properties, serves as a benchmark material for hydrogen storage, although its high cost and weight limit large-scale applications.
Overview of Amorphous Metals
Amorphous metals, also known as metallic glasses, exhibit a disordered atomic structure that enhances their hydrogen storage capacity through increased surface area and improved absorption kinetics compared to crystalline metals like palladium. These materials demonstrate superior resistance to hydrogen embrittlement and offer faster hydrogen diffusion rates, making them promising candidates for efficient hydrogen storage systems. Unlike palladium, which is costly and suffers from limited durability under repeated hydrogen cycles, amorphous metals provide a cost-effective and durable alternative with tunable properties for large-scale hydrogen storage applications.
Properties of Palladium in Hydrogen Storage
Palladium exhibits exceptional hydrogen storage properties due to its high hydrogen absorption capacity, reversible absorption-desorption cycles, and ability to form palladium hydride at relatively low pressures. Its face-centered cubic lattice structure enables rapid hydrogen diffusion, making it effective for applications requiring efficient hydrogen uptake and release. Palladium's stability and resistance to embrittlement during hydrogen cycling further enhance its suitability for long-term hydrogen storage technologies.
Hydrogen Absorption Mechanisms
Amorphous metals exhibit hydrogen absorption through a disordered atomic structure that facilitates rapid diffusion and uniform hydrogen distribution, enhancing storage capacity and kinetics. Palladium absorbs hydrogen via a well-defined lattice allowing hydrogen atoms to occupy interstitial sites, leading to phase transformations between a- and b-phases that influence storage efficiency. The amorphous metal's lack of grain boundaries reduces hydrogen trapping, whereas palladium's crystalline structure provides precise control over hydrogen solubility and release dynamics.
Storage Capacity Comparison
Amorphous metals exhibit a higher hydrogen storage capacity compared to palladium, with capacities often exceeding 2 wt% hydrogen due to their disordered atomic structure enhancing absorption sites. Palladium, while renowned for its excellent hydrogen absorption kinetics and selectivity, typically stores around 0.6 wt% hydrogen under similar conditions. The superior storage capacity of amorphous metals positions them as promising materials for efficient hydrogen storage in energy applications.
Kinetics and Reversibility
Amorphous metals exhibit superior hydrogen storage kinetics compared to palladium, enabling faster absorption and desorption rates due to their disordered atomic structure, which facilitates rapid hydrogen diffusion. Palladium, while known for excellent hydrogen solubility, often suffers from slower kinetics and potential hysteresis during absorption and release cycles, affecting its reversibility. The high reversibility of amorphous metals stems from their ability to maintain structural integrity without forming hydride phases that cause lattice expansion, resulting in more consistent cycling performance for hydrogen storage applications.
Structural Stability and Durability
Amorphous metals exhibit superior structural stability compared to palladium due to their disordered atomic arrangement, which resists hydrogen-induced lattice expansion and embrittlement. Palladium, while highly effective at hydrogen absorption, often suffers from phase transitions during cycling that degrade durability over time. The intrinsic resilience of amorphous metals to mechanical stress and hydrogen-induced strain enhances their longevity in hydrogen storage applications relative to crystalline palladium.
Cost and Scalability Considerations
Amorphous metals offer a cost-effective alternative to palladium for hydrogen storage due to lower raw material expenses and simpler manufacturing processes, enabling broader scalability. Palladium's high material cost and limited availability restrict its widespread adoption despite superior hydrogen absorption properties. Scaling amorphous metal production can leverage existing metallurgical infrastructure, improving economic feasibility for large-scale hydrogen storage applications.
Environmental Impact and Safety
Amorphous metals offer superior resistance to hydrogen embrittlement and exhibit high recyclability, reducing environmental hazards associated with metal degradation and waste. Palladium, while highly efficient at hydrogen absorption, poses risks due to its scarcity, energy-intensive extraction processes, and potential toxicity when released into ecosystems. Safety considerations favor amorphous metals for hydrogen storage as they demonstrate lower hydrogen leakage rates and improved structural stability under cyclic loading conditions compared to palladium-based systems.
Future Prospects and Research Directions
Amorphous metals exhibit significant potential for hydrogen storage due to their disordered atomic structure, which enhances hydrogen absorption capacity and kinetics compared to crystalline palladium. Future research aims to optimize alloy compositions and improve cycling stability to overcome current limitations in durability and cost. Exploring nanostructured amorphous metallic systems and integrating advanced computational modeling will accelerate the development of next-generation hydrogen storage materials.
Infographic: Amorphous metal vs Palladium for Hydrogen storage
 azmater.com
azmater.com