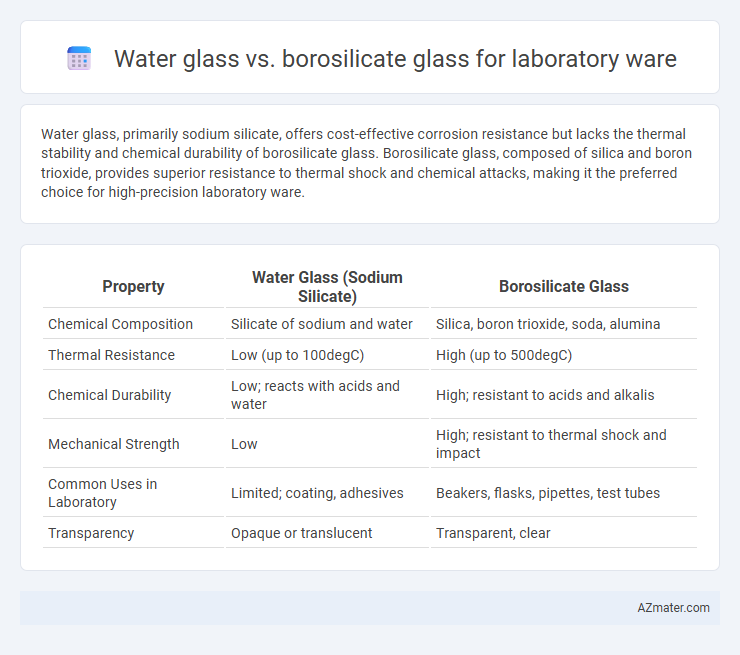
Water glass, primarily sodium silicate, offers cost-effective corrosion resistance but lacks the thermal stability and chemical durability of borosilicate glass. Borosilicate glass, composed of silica and boron trioxide, provides superior resistance to thermal shock and chemical attacks, making it the preferred choice for high-precision laboratory ware.
Table of Comparison
| Property | Water Glass (Sodium Silicate) | Borosilicate Glass |
|---|---|---|
| Chemical Composition | Silicate of sodium and water | Silica, boron trioxide, soda, alumina |
| Thermal Resistance | Low (up to 100degC) | High (up to 500degC) |
| Chemical Durability | Low; reacts with acids and water | High; resistant to acids and alkalis |
| Mechanical Strength | Low | High; resistant to thermal shock and impact |
| Common Uses in Laboratory | Limited; coating, adhesives | Beakers, flasks, pipettes, test tubes |
| Transparency | Opaque or translucent | Transparent, clear |
Introduction to Laboratory Glassware Materials
Water glass, also known as sodium silicate, is primarily used for coating and sealing purposes in laboratory settings but lacks the thermal resistance and chemical durability of borosilicate glass, which is the preferred material for laboratory glassware. Borosilicate glass contains silica and boron trioxide, providing enhanced resistance to thermal shock, chemical corrosion, and mechanical stress, making it ideal for beakers, flasks, and test tubes. Its high durability and stability under extreme conditions ensure precise and reliable experimental results in diverse scientific applications.
What is Water Glass?
Water glass, also known as sodium silicate, is a chemical compound used in laboratory ware primarily for its adhesive and sealing properties, and it differs significantly from borosilicate glass, which is favored for its thermal resistance and durability. While borosilicate glass withstands extreme temperature variations and chemical corrosion, water glass is a soluble liquid or gel form used to create coatings or as an immobilizing agent in lab processes. The choice between these materials depends on the laboratory application, with borosilicate glass serving as the standard for glassware like beakers and test tubes, and water glass used in specialized contexts requiring chemical sealing or preservation.
What is Borosilicate Glass?
Borosilicate glass is a type of laboratory glassware known for its exceptional thermal resistance and chemical durability, composed primarily of silica and boron trioxide. It withstands rapid temperature changes and corrosive substances, making it ideal for precise scientific experiments and high-temperature applications. Compared to water glass, borosilicate glass offers superior mechanical strength and resistance to thermal shock, ensuring reliable performance in rigorous laboratory environments.
Chemical Composition Comparison
Water glass, primarily composed of sodium silicate (Na2SiO3), features a high alkali content and is amorphous, while borosilicate glass contains silica (SiO2) and boron trioxide (B2O3) in a precise ratio, typically around 80% SiO2 and 13% B2O3, resulting in improved thermal and chemical resistance. The chemical composition of borosilicate glass reduces alkali mobility, enhancing durability against chemical corrosion and thermal shock compared to the more reactive sodium-rich water glass. These compositional differences make borosilicate glass the preferred choice for high-precision laboratory ware requiring stability under extreme conditions.
Thermal Resistance: Water Glass vs Borosilicate
Borosilicate glass offers superior thermal resistance compared to water glass, with a low coefficient of thermal expansion around 3.3 x 10^-6 /degC, enabling it to withstand rapid temperature changes without cracking. Water glass, primarily a sodium silicate solution, lacks the structural integrity when solidified to match borosilicate's heat tolerance, making it unsuitable for high-temperature lab applications. Laboratory ware made from borosilicate glass is preferred for thermal durability in experiments involving heating and cooling cycles.
Durability and Mechanical Strength
Water glass, primarily composed of sodium silicate, offers moderate durability but lower mechanical strength compared to borosilicate glass. Borosilicate glass contains boron trioxide, which enhances thermal resistance and mechanical toughness, making it ideal for laboratory ware exposed to rapid temperature changes and mechanical stress. Its superior resistance to chemical corrosion and higher tensile strength ensure longer service life and reliability in demanding laboratory environments.
Chemical Resistance and Reactivity
Borosilicate glass exhibits superior chemical resistance and low reactivity, making it ideal for laboratory ware exposed to harsh acids, bases, and temperature fluctuations. Water glass, or sodium silicate glass, has less chemical durability and is prone to leaching and surface degradation when in contact with strong chemicals. Laboratories prioritize borosilicate glass due to its robustness in maintaining integrity and preventing contamination during rigorous chemical procedures.
Cost and Availability in Laboratories
Water glass, or sodium silicate, is widely available and significantly less expensive than borosilicate glass, making it a cost-effective option for basic laboratory containers and storage. Borosilicate glass, known for its superior chemical resistance and thermal stability, often comes at a higher price and may have limited availability in smaller or less specialized laboratories. Laboratories prioritize borosilicate glass when durability and precision are critical despite its higher cost, while water glass serves as an economical alternative for general-purpose applications.
Common Applications of Each Glass Type
Water glass, or soda-lime glass, is commonly used for basic laboratory containers, such as beakers, test tubes, and bottles, due to its affordability and ease of shaping. Borosilicate glass features high thermal resistance and chemical durability, making it the preferred choice for precise lab equipment like volumetric flasks, condensers, and reaction vessels. Its ability to withstand rapid temperature changes and corrosive substances makes borosilicate indispensable in chemical synthesis and analytical applications.
Choosing the Right Glassware for Laboratory Use
Water glass, primarily composed of sodium silicate, offers excellent chemical resistance and is cost-effective for general laboratory applications involving alkalis and detergents, while borosilicate glass provides superior thermal resistance and mechanical strength, making it ideal for high-temperature experiments and handling corrosive chemicals. Selecting the right laboratory glassware depends on the specific application requirements such as temperature tolerance, chemical compatibility, and durability, with borosilicate glass preferred for most advanced scientific work due to its low thermal expansion and robustness. Understanding the distinct properties of water glass and borosilicate glass ensures optimal performance and safety in laboratory environments.
Infographic: Water glass vs Borosilicate glass for Laboratory ware
 azmater.com
azmater.com