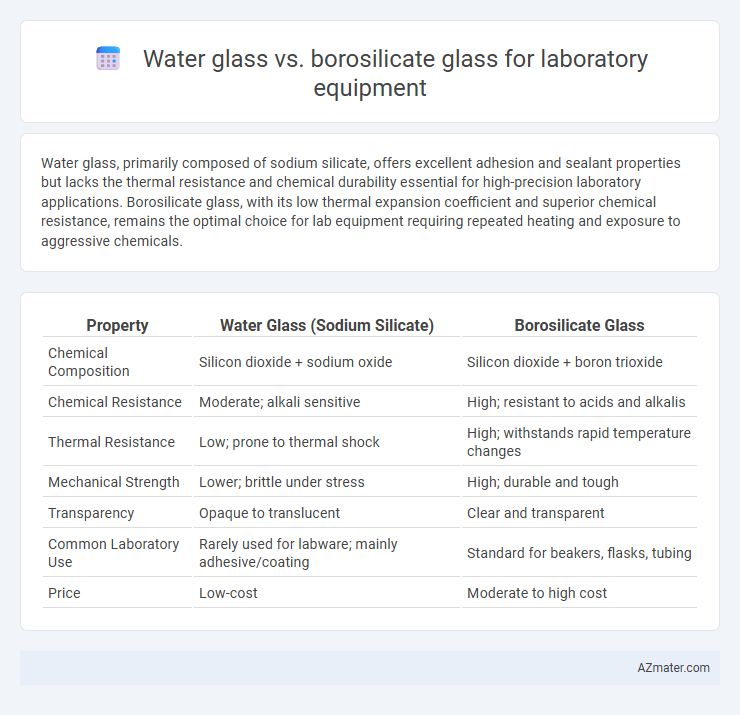
Water glass, primarily composed of sodium silicate, offers excellent adhesion and sealant properties but lacks the thermal resistance and chemical durability essential for high-precision laboratory applications. Borosilicate glass, with its low thermal expansion coefficient and superior chemical resistance, remains the optimal choice for lab equipment requiring repeated heating and exposure to aggressive chemicals.
Table of Comparison
| Property | Water Glass (Sodium Silicate) | Borosilicate Glass |
|---|---|---|
| Chemical Composition | Silicon dioxide + sodium oxide | Silicon dioxide + boron trioxide |
| Chemical Resistance | Moderate; alkali sensitive | High; resistant to acids and alkalis |
| Thermal Resistance | Low; prone to thermal shock | High; withstands rapid temperature changes |
| Mechanical Strength | Lower; brittle under stress | High; durable and tough |
| Transparency | Opaque to translucent | Clear and transparent |
| Common Laboratory Use | Rarely used for labware; mainly adhesive/coating | Standard for beakers, flasks, tubing |
| Price | Low-cost | Moderate to high cost |
Introduction to Laboratory Glassware Materials
Water glass, also known as sodium silicate, offers chemical resistance and affordability, making it suitable for specific non-critical laboratory equipment. Borosilicate glass is preferred for laboratory glassware due to its superior thermal resistance, low thermal expansion, and high chemical durability, ensuring reliability under harsh experimental conditions. Selecting the appropriate glass material impacts the accuracy, safety, and longevity of laboratory instruments.
What is Water Glass? Composition and Properties
Water glass, also known as sodium silicate, is an inorganic compound composed primarily of sodium oxide (Na2O) and silica (SiO2) in varying ratios, forming a glassy, soluble substance. Its key properties include high alkalinity, solubility in water, and excellent adhesive and sealing capabilities, making it useful for corrosion resistance and as a cement in laboratory settings. Unlike borosilicate glass, which is a durable, thermal-shock-resistant material made from silica and boron oxide, water glass is more of a chemical compound used for coatings or adhesives rather than structural glassware.
Borosilicate Glass: Features and Chemical Structure
Borosilicate glass, distinguished by its low thermal expansion coefficient and high chemical durability, is preferred for laboratory equipment requiring resistance to thermal shock and chemical corrosion. Its unique chemical structure consists of silica (SiO2) combined with boron trioxide (B2O3), which enhances heat resistance and reduces the tendency to crack under rapid temperature changes compared to ordinary water glass. This stable network of silicate and borate units makes borosilicate glass an ideal material for precise scientific experiments involving harsh chemicals and extreme temperature variations.
Thermal Resistance Comparison: Water Glass vs Borosilicate
Borosilicate glass offers superior thermal resistance compared to water glass, making it ideal for laboratory equipment subjected to rapid temperature changes. With a low coefficient of thermal expansion around 3.3 x 10^-6 /degC, borosilicate glass withstands thermal shock better than water glass, which has higher susceptibility to cracking under heat stress. This enhanced durability ensures borosilicate glass maintains structural integrity during sterilization and high-temperature experiments.
Chemical Durability in Laboratory Applications
Water glass, also known as sodium silicate, offers moderate chemical durability but is susceptible to alkaline hydrolysis and corrosion in acidic environments, limiting its use in laboratory applications requiring high chemical resistance. Borosilicate glass exhibits superior chemical durability due to its low coefficient of thermal expansion and resistance to acids, bases, and solvents, making it the preferred choice for most laboratory glassware such as beakers, flasks, and pipettes. Its ability to withstand thermal shock and aggressive chemicals ensures accurate experimental results and long-term usability in demanding laboratory conditions.
Mechanical Strength and Breakage Risks
Borosilicate glass exhibits superior mechanical strength and thermal resistance compared to water glass, making it more suitable for laboratory equipment subject to rapid temperature changes and mechanical stress. Its low coefficient of thermal expansion minimizes breakage risks during heating and cooling cycles, whereas water glass is more prone to fracture under similar conditions. The enhanced durability of borosilicate glass ensures greater safety and longevity in laboratory applications.
Cost Differences and Economic Considerations
Water glass, primarily made from sodium silicate, is significantly more affordable than borosilicate glass, which contains silica and boron oxide to withstand thermal stress. Laboratory equipment crafted from borosilicate glass demands higher initial investment due to its superior durability and resistance to chemical corrosion, reducing long-term replacement costs. Economic considerations favor water glass for budget-sensitive applications, but borosilicate glass offers cost-efficiency over time where thermal shock resistance and longevity are critical.
Suitability for High-Temperature Procedures
Borosilicate glass offers superior thermal resistance with a high melting point around 820degC, making it ideal for high-temperature laboratory procedures such as heating and sterilization. Water glass, primarily composed of sodium silicate, lacks the thermal stability required for extreme heat, often leading to deformation or breakage under intense thermal stress. Therefore, borosilicate glass is preferred for lab equipment used in rigorous thermal applications due to its low coefficient of thermal expansion and enhanced durability.
Safety and Environmental Concerns
Water glass, or sodium silicate, is less durable and chemically resistant compared to borosilicate glass, making it less safe for laboratory use due to potential breakage and chemical reactions. Borosilicate glass offers superior thermal resistance and chemical stability, reducing the risk of hazardous spills and glass breakage, thereby enhancing laboratory safety. Environmentally, borosilicate glass is more sustainable due to its longer lifespan and recyclability, while water glass may contribute to environmental pollution if disposed of improperly.
Conclusion: Selecting the Right Glass for Your Laboratory
Water glass, primarily composed of sodium silicate, offers affordability and corrosion resistance but lacks the thermal and chemical durability of borosilicate glass. Borosilicate glass excels in laboratory applications due to its low thermal expansion, high chemical resistance, and durability under extreme temperatures. Selecting borosilicate glass ensures reliable performance and longevity in demanding experimental conditions, making it the preferred choice for most laboratory equipment.
Infographic: Water glass vs Borosilicate glass for Laboratory equipment
 azmater.com
azmater.com