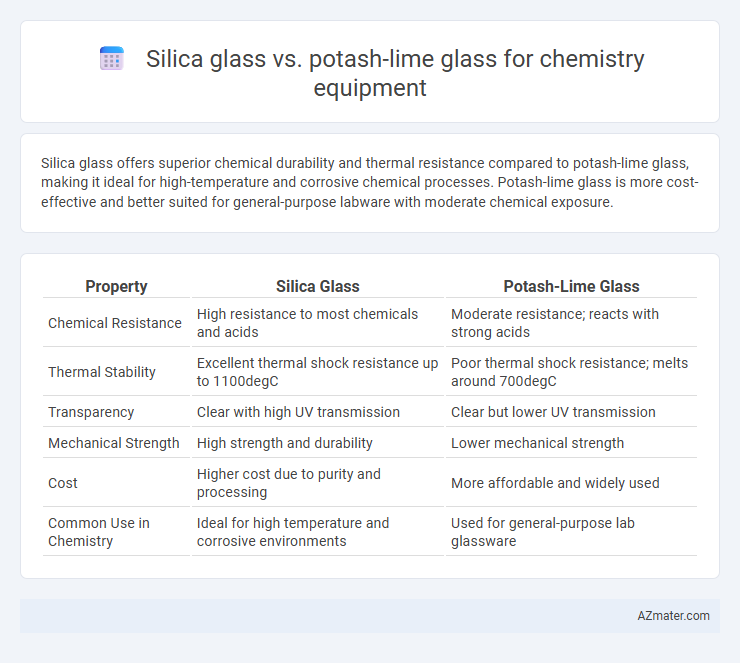
Silica glass offers superior chemical durability and thermal resistance compared to potash-lime glass, making it ideal for high-temperature and corrosive chemical processes. Potash-lime glass is more cost-effective and better suited for general-purpose labware with moderate chemical exposure.
Table of Comparison
| Property | Silica Glass | Potash-Lime Glass |
|---|---|---|
| Chemical Resistance | High resistance to most chemicals and acids | Moderate resistance; reacts with strong acids |
| Thermal Stability | Excellent thermal shock resistance up to 1100degC | Poor thermal shock resistance; melts around 700degC |
| Transparency | Clear with high UV transmission | Clear but lower UV transmission |
| Mechanical Strength | High strength and durability | Lower mechanical strength |
| Cost | Higher cost due to purity and processing | More affordable and widely used |
| Common Use in Chemistry | Ideal for high temperature and corrosive environments | Used for general-purpose lab glassware |
Introduction to Laboratory Glass Types
Silica glass, known for its exceptional thermal resistance and chemical inertness, is ideal for high-precision laboratory applications requiring durability under extreme temperature changes. Potash-lime glass, more economical and commonly used, offers adequate chemical resistance and is suitable for general-purpose laboratory glassware such as beakers and flasks. Choosing between silica and potash-lime glass depends on the specific experimental conditions, including temperature tolerance and chemical reactivity.
Chemical Composition of Silica Glass
Silica glass, primarily composed of silicon dioxide (SiO2), offers superior chemical resistance and thermal stability compared to potash-lime glass, which contains mixtures of sodium oxide (Na2O), potassium oxide (K2O), calcium oxide (CaO), and silicon dioxide. The high purity and consistent SiO2 content in silica glass minimize chemical reactions and leaching during laboratory experiments, making it ideal for handling aggressive chemicals. Potash-lime glass, with its variable alkali and alkaline earth metal oxides, is more susceptible to corrosion and lower thermal tolerance, limiting its use in high-precision chemical applications.
Chemical Composition of Potash-Lime Glass
Potash-lime glass comprises primarily silica (SiO2) around 70-74%, with significant amounts of potassium oxide (K2O) and sodium oxide (Na2O) typically totaling 12-16%, and calcium oxide (CaO) at about 5-11%, which enhance its thermal and chemical durability. The presence of alkali metals like potassium and sodium in potash-lime glass increases its susceptibility to chemical attack compared to silica glass, making it less resistant to strong acids and bases commonly used in chemical laboratories. Silica glass, composed almost entirely of pure silicon dioxide, offers superior chemical inertness and thermal stability, making it the preferred choice for high-precision chemical equipment requiring resistance to aggressive reagents.
Thermal Resistance: Silica vs Potash-Lime Glass
Silica glass exhibits significantly higher thermal resistance compared to potash-lime glass, making it ideal for high-temperature chemistry applications requiring rapid heating and cooling cycles. Potash-lime glass tends to soften and deform at temperatures above 600degC, whereas silica glass can withstand temperatures exceeding 1100degC without losing structural integrity. The superior thermal shock resistance of silica glass reduces the risk of cracking during sudden temperature changes in laboratory settings.
Chemical Durability and Reactivity
Silica glass exhibits superior chemical durability and minimal reactivity, making it highly resistant to acids, alkalis, and organic solvents, which is critical for precise chemical experiments. Potash-lime glass, while more affordable, has lower chemical resistance and can leach alkaline ions, potentially contaminating reactive solutions. The high purity and inert nature of silica glass reduce the risk of chemical interaction, ensuring better long-term stability in laboratory applications.
Mechanical Strength and Fragility
Silica glass exhibits superior mechanical strength and higher resistance to thermal shock compared to potash-lime glass, making it less prone to cracking under rapid temperature changes. Potash-lime glass is more fragile due to its lower tensile strength and greater susceptibility to mechanical stress, often leading to easier breakage during laboratory handling. The enhanced durability of silica glass ensures reliability in chemical experiments requiring repeated heating and cooling cycles.
Optical Clarity and Transparency
Silica glass offers superior optical clarity and transparency compared to potash-lime glass, making it ideal for precise chemical optical measurements and experiments. Its low impurity levels result in minimal light absorption and high UV transmission, crucial for spectroscopy and photochemical reactions. In contrast, potash-lime glass exhibits lower optical performance and reduced UV transparency, limiting its use in advanced optical applications within chemistry labs.
Common Laboratory Applications
Silica glass offers superior chemical resistance and high thermal stability, making it ideal for high-temperature reactions and corrosive chemicals commonly encountered in advanced laboratory setups. Potash-lime glass is preferred for general laboratory applications due to its affordability and adequate resistance to moderate chemical exposure, suitable for routine glassware like beakers and flasks. Both types play essential roles in chemistry labs, with silica glass excelling in precision experiments and potash-lime glass used for everyday tasks.
Cost and Availability
Silica glass, known for its high thermal and chemical resistance, is significantly more expensive and less widely available compared to potash-lime glass, which is more affordable and commonly used in standard laboratory glassware. Potash-lime glass offers sufficient durability for routine chemical applications, making it the cost-effective choice for most laboratories. The premium cost of silica glass often limits its use to specialized equipment requiring superior heat and chemical resistance.
Choosing the Right Glass for Chemical Equipment
Silica glass offers superior thermal resistance and chemical inertness, making it ideal for highly corrosive chemicals and high-temperature applications in chemistry equipment. Potash-lime glass, while more affordable and easier to shape, lacks the chemical durability and heat tolerance of silica glass, limiting its use to less demanding laboratory tasks. Selecting the right glass depends on the required chemical resistance, temperature stability, and budget constraints for safe and effective laboratory operations.
Infographic: Silica glass vs Potash-lime glass for Chemistry equipment
 azmater.com
azmater.com