Potash-lime glass offers cost-effective durability for general laboratory apparatus, while borosilicate glass provides superior thermal resistance and chemical stability essential for high-precision experiments. Borosilicate's low thermal expansion makes it ideal for applications involving rapid temperature changes or corrosive chemicals.
Table of Comparison
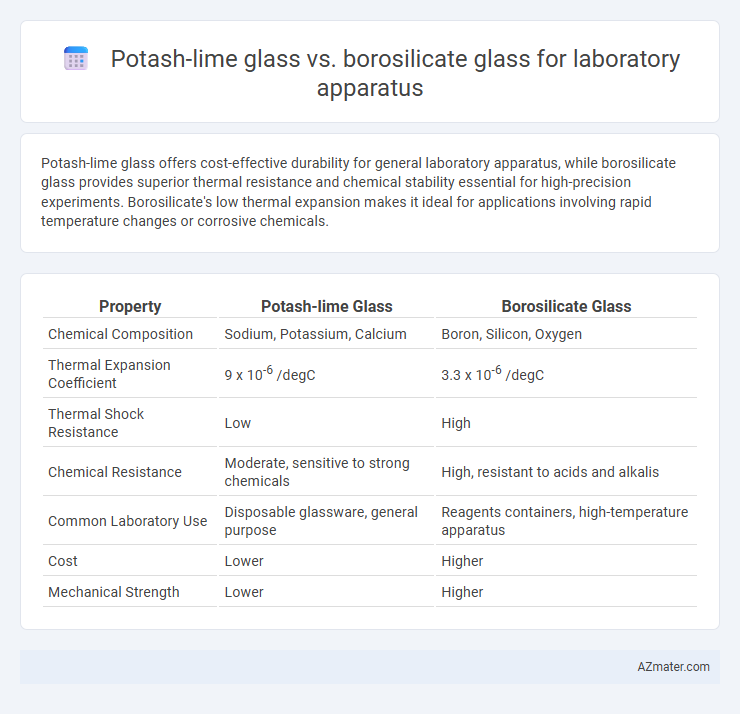
| Property | Potash-lime Glass | Borosilicate Glass |
|---|---|---|
| Chemical Composition | Sodium, Potassium, Calcium | Boron, Silicon, Oxygen |
| Thermal Expansion Coefficient | 9 x 10-6 /degC | 3.3 x 10-6 /degC |
| Thermal Shock Resistance | Low | High |
| Chemical Resistance | Moderate, sensitive to strong chemicals | High, resistant to acids and alkalis |
| Common Laboratory Use | Disposable glassware, general purpose | Reagents containers, high-temperature apparatus |
| Cost | Lower | Higher |
| Mechanical Strength | Lower | Higher |
Introduction to Laboratory Glassware Materials
Potash-lime glass, primarily composed of silica, potash, and lime, offers affordability and good optical clarity but has lower thermal and chemical resistance compared to borosilicate glass, which contains boron oxide in addition to silica, enhancing durability and thermal shock resistance. Borosilicate glass is the preferred material for laboratory apparatus due to its ability to withstand high temperatures and corrosive chemicals without deformation or degradation. The choice between potash-lime and borosilicate glass depends on the specific laboratory application requirements, with borosilicate dominating in precision and high-stress environments.
Composition of Potash-Lime Glass
Potash-lime glass, commonly used in laboratory apparatus, primarily consists of silica (SiO2), potassium oxide (K2O), lime (CaO), and smaller amounts of soda (Na2O) and alumina (Al2O3). The high potassium oxide content reduces the melting temperature and improves the glass's resistance to water and chemical corrosion compared to soda-lime glass. This composition results in lower thermal resistance and chemical durability than borosilicate glass, making potash-lime glass less suitable for high-temperature or harsh chemical applications in labs.
Composition of Borosilicate Glass
Borosilicate glass, widely used in laboratory apparatus, is composed primarily of silica (SiO2) around 70-80%, boron oxide (B2O3) about 7-13%, along with smaller amounts of sodium oxide (Na2O) and aluminum oxide (Al2O3), which enhance its thermal resistance and chemical durability. In contrast to Potash-lime glass, which contains higher levels of potassium oxide (K2O) and calcium oxide (CaO), borosilicate glass exhibits superior resistance to thermal shock and corrosive chemicals due to the boron oxide content. This composition makes borosilicate glass ideal for laboratory applications requiring high temperature stability and chemical inertness.
Thermal Resistance: Potash-Lime vs Borosilicate Glass
Borosilicate glass exhibits superior thermal resistance compared to potash-lime glass, with a low coefficient of thermal expansion around 3.3 x 10-6 /degC, allowing it to withstand rapid temperature changes and thermal shock. Potash-lime glass, having a higher coefficient of thermal expansion approximately 9 x 10-6 /degC, is more prone to cracking under thermal stress. Laboratory apparatus made from borosilicate glass is preferred for applications requiring heat resistance and durability in extreme temperature fluctuations.
Chemical Durability Comparison
Potash-lime glass exhibits lower chemical durability compared to borosilicate glass, making it more susceptible to attack by alkalis and acids in laboratory conditions. Borosilicate glass contains boron oxide, which enhances its resistance to chemical corrosion and thermal shock, ensuring longevity and reliability in chemical experiments. This increased chemical durability of borosilicate glass makes it the preferred choice for laboratory apparatus exposed to aggressive reagents.
Mechanical Strength and Shock Resistance
Potash-lime glass exhibits moderate mechanical strength and lower thermal shock resistance, making it more susceptible to breakage during rapid temperature changes in laboratory settings. Borosilicate glass offers superior mechanical strength and exceptional resistance to thermal shock, ensuring durability and safety when exposed to sudden temperature fluctuations. Laboratories requiring robust apparatus for heating and cooling processes commonly prefer borosilicate glass for its enhanced performance and longevity.
Cost and Economic Considerations
Potash-lime glass is significantly cheaper than borosilicate glass, making it a cost-effective choice for general laboratory apparatus where resistance to thermal shock and chemical corrosion is not critical. Borosilicate glass offers superior durability, thermal resistance, and chemical stability, justifying its higher price in applications requiring precision and longevity. Laboratories with tight budgets often opt for potash-lime glass for disposable or low-risk equipment, while borosilicate glass is preferred for reusable, high-performance glassware despite the higher initial investment.
Common Laboratory Applications
Potash-lime glass is widely used for standard laboratory apparatus such as beakers, test tubes, and petri dishes due to its affordability and good chemical resistance against acids and alkalis. Borosilicate glass, preferred for high-precision laboratory equipment like volumetric flasks, pipettes, and condensers, offers superior thermal resistance and durability under rapid temperature changes. Laboratories handling heating, cooling, or strong chemicals often choose borosilicate glass for reliability in demanding applications.
Safety and Handling Differences
Potash-lime glass, commonly used for disposable laboratory apparatus, offers lower chemical resistance and thermal shock resistance compared to borosilicate glass, which excels in withstanding high temperatures and aggressive chemicals. Borosilicate glass's superior durability and resistance reduce the risk of breakage and chemical leakage, ensuring safer handling during rigorous laboratory procedures. Proper use of borosilicate glass minimizes hazards in heating, cooling, and chemical exposure, making it the preferred choice for reusable and high-safety glassware.
Choosing the Right Glass for Laboratory Use
Potash-lime glass is economical and suitable for general lab applications with lower thermal stress, offering good chemical resistance to acids and alkalis but limited resistance to sudden temperature changes. Borosilicate glass, such as Pyrex, provides superior thermal shock resistance, high durability, and excellent chemical inertness, making it ideal for experiments involving rapid heating and cooling or corrosive substances. Selecting the right glass depends on the specific laboratory tasks, where borosilicate glass is preferred for high-precision and high-temperature workflows, while potash-lime glass suffices for routine, low-stress applications.
Infographic: Potash-lime glass vs Borosilicate glass for Laboratory apparatus
 azmater.com
azmater.com