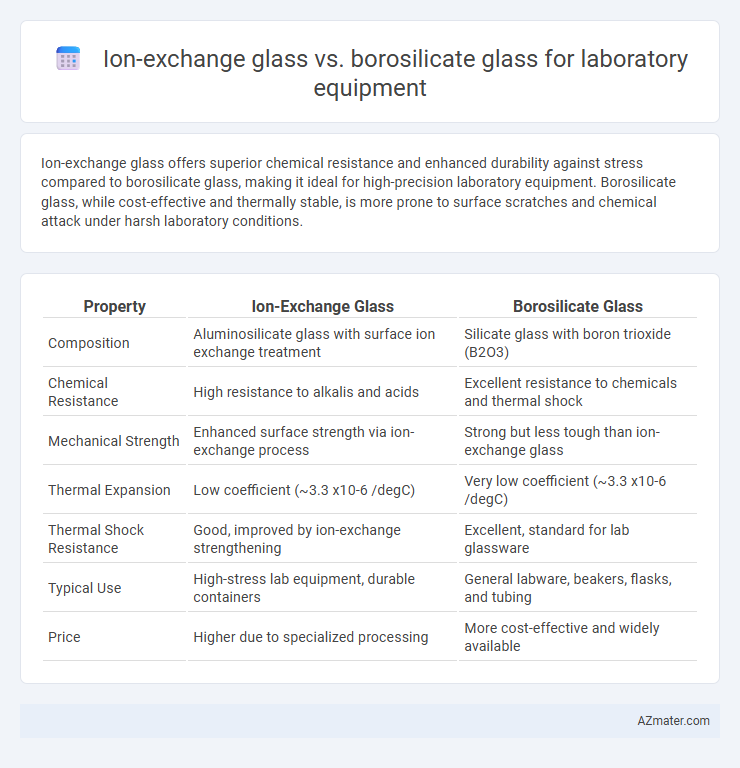
Ion-exchange glass offers superior chemical resistance and enhanced durability against stress compared to borosilicate glass, making it ideal for high-precision laboratory equipment. Borosilicate glass, while cost-effective and thermally stable, is more prone to surface scratches and chemical attack under harsh laboratory conditions.
Table of Comparison
| Property | Ion-Exchange Glass | Borosilicate Glass |
|---|---|---|
| Composition | Aluminosilicate glass with surface ion exchange treatment | Silicate glass with boron trioxide (B2O3) |
| Chemical Resistance | High resistance to alkalis and acids | Excellent resistance to chemicals and thermal shock |
| Mechanical Strength | Enhanced surface strength via ion-exchange process | Strong but less tough than ion-exchange glass |
| Thermal Expansion | Low coefficient (~3.3 x10-6 /degC) | Very low coefficient (~3.3 x10-6 /degC) |
| Thermal Shock Resistance | Good, improved by ion-exchange strengthening | Excellent, standard for lab glassware |
| Typical Use | High-stress lab equipment, durable containers | General labware, beakers, flasks, and tubing |
| Price | Higher due to specialized processing | More cost-effective and widely available |
Introduction to Laboratory Glassware Materials
Ion-exchange glass offers enhanced chemical durability and resistance to alkali attack compared to borosilicate glass, making it suitable for high-purity laboratory applications. Borosilicate glass, known for its excellent thermal resistance and mechanical strength, remains a cost-effective standard material for general labware such as beakers and flasks. Selection between ion-exchange and borosilicate glass depends on specific laboratory requirements including chemical exposure, temperature stability, and mechanical stress tolerance.
What is Ion-Exchange Glass?
Ion-exchange glass is a type of chemically strengthened glass created by replacing smaller ions in the glass surface with larger ions through an ion-exchange process, enhancing its strength and resistance to stress. Compared to borosilicate glass, which is valued for its thermal stability and chemical resistance, ion-exchange glass offers superior mechanical durability and scratch resistance, making it ideal for laboratory equipment exposed to physical impact. This advanced glass type combines resilience with transparency, ensuring both safety and clarity in demanding laboratory environments.
What is Borosilicate Glass?
Borosilicate glass is a type of laboratory glass known for its exceptional thermal resistance and chemical durability, composed primarily of silica and boron trioxide. It can withstand rapid temperature changes without cracking, making it ideal for applications involving heating and cooling cycles. Compared to ion-exchange glass, borosilicate glass offers superior chemical resistance and mechanical strength, ensuring long-lasting performance in demanding laboratory environments.
Chemical Resistance: Ion-Exchange vs Borosilicate
Ion-exchange glass exhibits enhanced chemical resistance compared to borosilicate glass due to its surface modification that replaces sodium ions with larger potassium ions, reducing ion exchange and leaching in acidic and alkaline solutions. Borosilicate glass is known for its general chemical durability, but it is more prone to surface degradation under harsh chemical exposure, especially with strong alkalis and hydrofluoric acid. The superior resistance of ion-exchange glass makes it preferable for laboratory equipment requiring prolonged exposure to aggressive reagents without compromising structural integrity.
Thermal Stability and Temperature Limits
Ion-exchange glass offers superior thermal stability with enhanced resistance to sudden temperature changes, supporting temperature limits up to approximately 450degC. Borosilicate glass is known for its excellent thermal shock resistance and can typically withstand continuous use temperatures around 500degC but is more prone to gradual deformation under prolonged heat. Choosing between ion-exchange and borosilicate glass depends on specific laboratory needs for thermal durability and operating temperature ranges.
Mechanical Strength and Durability Comparison
Ion-exchange glass exhibits superior mechanical strength and enhanced durability compared to borosilicate glass due to its surface compression layer created during the ion-exchange process, making it more resistant to scratches and breakage under stress. Borosilicate glass, while chemically resistant and thermally stable, has lower tensile strength and is more prone to mechanical damage and chipping in high-impact laboratory environments. The enhanced toughness of ion-exchange glass results in longer-lasting laboratory equipment ideal for applications requiring frequent handling and mechanical resilience.
Cost and Economic Considerations
Ion-exchange glass for laboratory equipment offers enhanced chemical durability and resistance to thermal shock compared to borosilicate glass, but its manufacturing process results in a higher initial cost. Borosilicate glass remains the more economical choice due to its widespread availability and lower production expenses, making it suitable for routine laboratory applications. When budgeting, factors such as long-term durability, potential replacements, and specific experimental requirements should guide the decision between the higher upfront investment in ion-exchange glass versus the cost-effectiveness of borosilicate glass.
Common Laboratory Applications
Ion-exchange glass offers enhanced chemical durability and resistance to alkali attack, making it ideal for high-precision analytical applications and long-term storage of aggressive chemicals in laboratory settings. Borosilicate glass is widely favored for routine laboratory equipment such as beakers, flasks, and condensers due to its excellent thermal shock resistance and compatibility with most reagents. Common laboratory applications utilize ion-exchange glass primarily in ion chromatography and pH-sensitive measurements, while borosilicate glass remains the standard for heating, mixing, and general glassware needs.
Safety Aspects and Handling
Ion-exchange glass offers enhanced chemical resistance and greater mechanical strength compared to borosilicate glass, reducing the risk of breakage in laboratory settings. Borosilicate glass, while durable and thermally stable, is more prone to surface scratches that can lead to stress points and potential fractures during handling. Prioritizing ion-exchange glass in laboratory equipment improves safety by minimizing glassware failure and offers easier maintenance with less susceptibility to damage from everyday use.
Choosing the Right Glass Type for Your Lab
Ion-exchange glass offers enhanced chemical resistance and improved durability compared to borosilicate glass, making it ideal for laboratories handling aggressive chemicals and requiring long-lasting equipment. Borosilicate glass remains a versatile, cost-effective choice with excellent thermal resistance and good mechanical strength, suitable for general laboratory applications. Selecting the right glass type depends on the specific laboratory tasks, chemical exposure, and budget considerations to optimize performance and safety.
Infographic: Ion-exchange glass vs Borosilicate glass for Laboratory equipment
 azmater.com
azmater.com