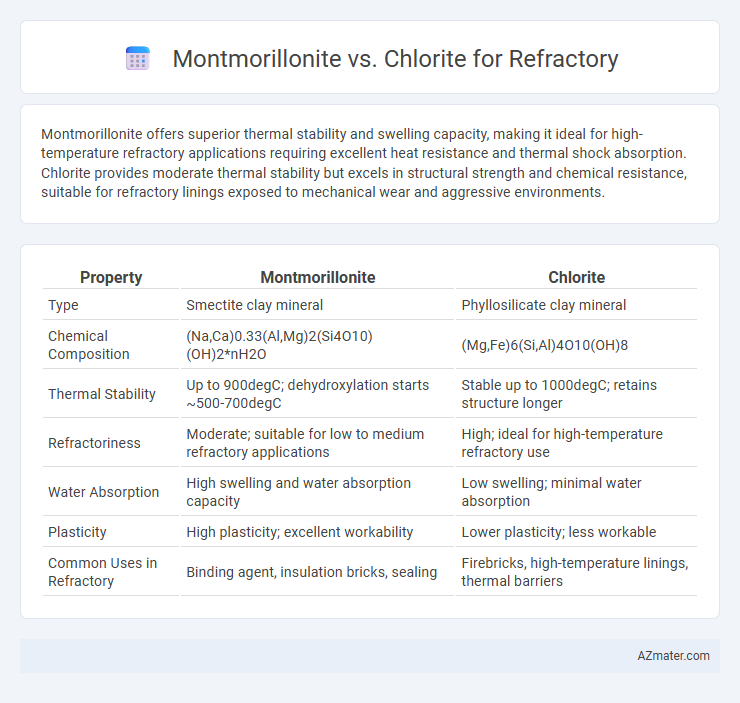
Montmorillonite offers superior thermal stability and swelling capacity, making it ideal for high-temperature refractory applications requiring excellent heat resistance and thermal shock absorption. Chlorite provides moderate thermal stability but excels in structural strength and chemical resistance, suitable for refractory linings exposed to mechanical wear and aggressive environments.
Table of Comparison
| Property | Montmorillonite | Chlorite |
|---|---|---|
| Type | Smectite clay mineral | Phyllosilicate clay mineral |
| Chemical Composition | (Na,Ca)0.33(Al,Mg)2(Si4O10)(OH)2*nH2O | (Mg,Fe)6(Si,Al)4O10(OH)8 |
| Thermal Stability | Up to 900degC; dehydroxylation starts ~500-700degC | Stable up to 1000degC; retains structure longer |
| Refractoriness | Moderate; suitable for low to medium refractory applications | High; ideal for high-temperature refractory use |
| Water Absorption | High swelling and water absorption capacity | Low swelling; minimal water absorption |
| Plasticity | High plasticity; excellent workability | Lower plasticity; less workable |
| Common Uses in Refractory | Binding agent, insulation bricks, sealing | Firebricks, high-temperature linings, thermal barriers |
Introduction to Montmorillonite and Chlorite
Montmorillonite, a smectite group clay mineral, is characterized by its high cation exchange capacity and excellent thermal stability, making it suitable for refractory applications requiring heat resistance and insulation. Chlorite, a phyllosilicate mineral, offers notable thermal shock resistance and dimensional stability due to its layered structure and magnesium-rich composition. Comparing Montmorillonite and Chlorite highlights their distinct mineralogical properties influencing performance in high-temperature refractory environments.
Chemical Composition Differences
Montmorillonite consists primarily of a 2:1 layered structure with a high content of silica (SiO2) and alumina (Al2O3), along with exchangeable cations like Na+, Ca2+, and Mg2+, which contribute to its swelling properties and thermal stability in refractory applications. Chlorite features a 2:1:1 layered structure, incorporating an additional brucite-like layer rich in magnesium hydroxide (Mg(OH)2), resulting in higher MgO content and lower silica compared to montmorillonite, affecting its thermal behavior and refractory performance. These chemical composition differences influence their thermal expansion, heat resistance, and suitability for specific refractory environments requiring either swelling capacity or structural stability under high temperatures.
Crystal Structure and Morphology
Montmorillonite exhibits a 2:1 phyllosilicate crystal structure with expansive interlayer spacing, allowing for high cation exchange capacity and swelling properties, which enhance its thermal shock resistance in refractory applications. Chlorite has a 2:1:1 layered structure featuring a brucite-like sheet that imparts greater dimensional stability and resistance to high-temperature deformation. Morphologically, montmorillonite consists of thin, plate-like particles with high surface area, while chlorite forms more rigid, thicker flakes, influencing their performance in refractory composites.
Thermal Stability in Refractory Applications
Montmorillonite exhibits excellent thermal stability up to approximately 700degC, making it suitable for moderate refractory applications where thermal shock resistance and plasticity are required. Chlorite offers superior thermal stability, retaining structural integrity beyond 900degC, which enhances its performance in high-temperature refractory environments. The choice between montmorillonite and chlorite depends on the specific temperature range and durability demands of the refractory system.
Mechanical Properties Comparison
Montmorillonite exhibits superior thermal shock resistance and higher plasticity compared to chlorite, making it more effective in maintaining mechanical integrity under fluctuating temperatures in refractory applications. Chlorite offers greater compressive strength due to its dense crystal structure but has lower resistance to thermal cycling, which can lead to microcracking and reduced durability. The mechanical properties of montmorillonite, including enhanced elasticity and tensile strength, contribute to improved refractory lifespans in high-stress environments.
Effect on Refractory Material Performance
Montmorillonite enhances refractory material performance by improving plasticity and thermal shock resistance due to its high cation exchange capacity and swelling properties. Chlorite contributes to refractory stability through its laminar structure, which offers better dimensional stability and resistance to chemical attack at high temperatures. The combination of montmorillonite's adaptability and chlorite's structural rigidity results in optimized thermal insulation and mechanical strength in refractory applications.
Reaction with High-Temperature Phases
Montmorillonite exhibits strong cation exchange capacity and dehydrates to form metakaolin, which reacts with high-temperature phases to produce spinel and mullite, enhancing refractory strength and thermal stability. Chlorite dehydrates to release MgO and FeO, facilitating the formation of complex silicate phases like olivine and pyroxene under high temperatures, providing thermal shock resistance but lower refractory strength compared to mullite-bearing materials. The selection between montmorillonite and chlorite in refractory applications depends on the desired balance between mechanical strength and resistance to thermal cycling due to their distinct high-temperature reactions and phase formations.
Cost and Availability for Industrial Use
Montmorillonite offers higher availability and lower cost compared to chlorite, making it more economical for industrial refractory applications. Industrial-grade montmorillonite is widely sourced from major deposits worldwide, ensuring steady supply and competitive pricing. Chlorite, while providing specific thermal stability benefits, is less abundant and typically commands a higher price, limiting its cost-effectiveness in large-scale refractory manufacturing.
Environmental and Safety Considerations
Montmorillonite offers superior thermal stability and low toxicity, making it a safer choice for refractory applications with minimal environmental impact during processing and use. Chlorite, while durable, contains higher levels of heavy metals that pose potential health risks and generate hazardous dust, demanding stringent safety measures. Selecting Montmorillonite can reduce occupational exposure and environmental contamination, aligning with sustainable refractory manufacturing practices.
Conclusion: Choosing the Right Clay for Refractories
Montmorillonite offers superior thermal shock resistance and swelling capacity, making it ideal for high-temperature refractory applications requiring flexibility and durability. Chlorite provides enhanced chemical stability and a higher melting point, suitable for refractories exposed to aggressive environments and prolonged thermal exposure. Selecting the right clay depends on balancing thermal resilience and chemical durability to optimize refractory performance in specific industrial conditions.
Infographic: Montmorillonite vs Chlorite for Refractory
 azmater.com
azmater.com