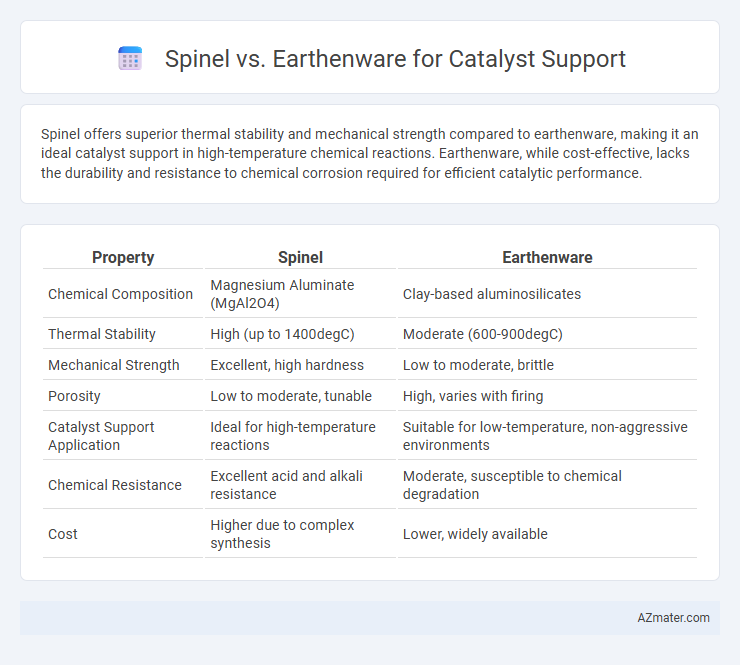
Spinel offers superior thermal stability and mechanical strength compared to earthenware, making it an ideal catalyst support in high-temperature chemical reactions. Earthenware, while cost-effective, lacks the durability and resistance to chemical corrosion required for efficient catalytic performance.
Table of Comparison
| Property | Spinel | Earthenware |
|---|---|---|
| Chemical Composition | Magnesium Aluminate (MgAl2O4) | Clay-based aluminosilicates |
| Thermal Stability | High (up to 1400degC) | Moderate (600-900degC) |
| Mechanical Strength | Excellent, high hardness | Low to moderate, brittle |
| Porosity | Low to moderate, tunable | High, varies with firing |
| Catalyst Support Application | Ideal for high-temperature reactions | Suitable for low-temperature, non-aggressive environments |
| Chemical Resistance | Excellent acid and alkali resistance | Moderate, susceptible to chemical degradation |
| Cost | Higher due to complex synthesis | Lower, widely available |
Introduction to Catalyst Supports
Catalyst supports such as spinel and earthenware play crucial roles in enhancing catalytic performance by providing a stable surface for active sites. Spinel, characterized by its robust crystal structure and high thermal stability, offers superior mechanical strength and resistance to sintering under harsh reaction conditions. Earthenware, while cost-effective and widely available, typically has lower thermal stability and surface area, limiting its effectiveness in high-temperature catalytic applications.
Overview of Spinel Materials
Spinel materials, characterized by their AB2O4 crystalline structure, offer exceptional thermal stability and mechanical strength, making them ideal for catalyst support applications. Their unique oxygen ion conductivity and resistance to sintering enhance the dispersion and longevity of active catalytic phases compared to traditional earthenware supports. These properties result in improved catalyst performance, especially in high-temperature reactions such as oxidative coupling and reforming processes.
Overview of Earthenware Materials
Earthenware materials, primarily composed of natural clays like kaolinite and illite, offer a porous and cost-effective support for catalysts, promoting high dispersion of active phases. These materials typically exhibit lower thermal stability and mechanical strength compared to spinel supports but provide superior ion-exchange capacity and surface hydroxyl groups beneficial for catalytic reactions. Their ease of shaping and abundance make earthenware an attractive option for large-scale catalyst support applications despite limitations under high-temperature conditions.
Structural Differences: Spinel vs Earthenware
Spinel catalysts exhibit a distinct crystalline structure characterized by a cubic close-packed oxygen lattice with metal ions occupying both tetrahedral and octahedral sites, enhancing thermal stability and catalytic activity. In contrast, earthenware, composed mainly of amorphous or poorly crystalline aluminosilicate materials, lacks the ordered lattice framework, resulting in lower mechanical strength and limited high-temperature performance. The structural rigidity and uniformity of spinel frameworks facilitate superior dispersion and anchoring of active catalytic species compared to the porous and heterogeneous matrix of earthenware supports.
Thermal Stability Comparison
Spinel structures exhibit superior thermal stability compared to earthenware when used as catalyst supports, maintaining structural integrity at temperatures exceeding 1000degC. Earthenware, composed mainly of clay-based ceramics, tends to degrade or undergo phase changes at elevated temperatures around 900degC, limiting its application in high-temperature catalytic processes. The high melting point and resistance to sintering of spinel materials ensure prolonged catalyst performance under harsh thermal conditions.
Porosity and Surface Area Analysis
Spinel catalysts exhibit higher thermal stability and enhanced porosity compared to earthenware, which typically shows lower surface area and pore volume due to its dense ceramic matrix. Surface area analysis via BET indicates that spinel supports have a greater specific surface area, promoting improved catalytic activity by providing more active sites for reaction. Porosity measurements reveal that spinels possess interconnected mesopores facilitating better mass transfer, whereas earthenware's microporous structure limits reactant diffusion and reduces overall catalytic efficiency.
Chemical Compatibility with Catalysts
Spinel exhibits superior chemical compatibility with catalysts due to its stable crystalline structure and high resistance to acidic and basic environments, preventing unwanted reactions or catalyst poisoning during reactions. Earthenware, composed primarily of porous clay minerals, may interact chemically with certain catalysts, leading to potential contamination or diminished catalytic activity. The inherent inertness and thermal stability of spinel make it a preferred support material for advanced catalytic processes requiring stringent chemical compatibility.
Cost and Scalability Considerations
Spinel catalysts exhibit higher initial material costs due to complex synthesis but offer superior thermal stability and longevity, reducing long-term replacement expenses. Earthenware, made from abundant natural clays, costs significantly less and is easier to produce at scale, making it attractive for large-volume applications despite lower durability. The choice between spinel and earthenware hinges on balancing upfront investment with maintenance frequency and scale-dependent production economics.
Performance in Industrial Applications
Spinel catalysts offer superior thermal stability and resistance to sintering compared to earthenware, making them more effective in high-temperature industrial processes such as catalytic reforming and oxidation reactions. The enhanced surface area and mechanical robustness of spinel structures result in improved catalytic activity and longer operational lifespan under harsh conditions. Earthenware, while cost-effective, typically exhibits lower thermal conductivity and chemical durability, limiting its efficiency and durability as a catalyst support in demanding industrial environments.
Conclusion: Choosing the Right Support
Spinel catalysts offer superior thermal stability, mechanical strength, and resistance to sintering compared to earthenware, making them ideal for high-temperature applications. Earthenware supports are more cost-effective and easier to manufacture but generally lack the durability and chemical resistance required for demanding catalytic processes. Selecting the appropriate support depends on operational conditions, with spinel favored for longevity and performance in harsh environments.
Infographic: Spinel vs Earthenware for Catalyst support
 azmater.com
azmater.com