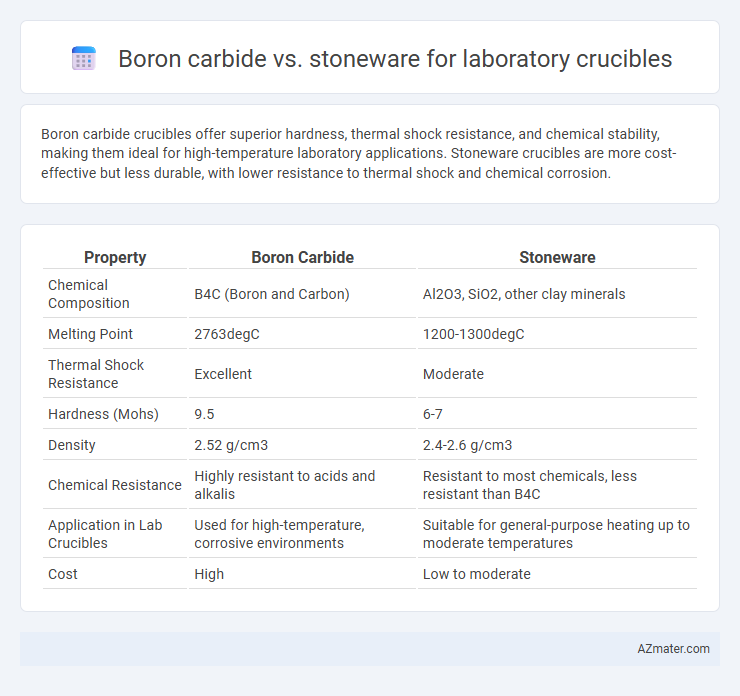
Boron carbide crucibles offer superior hardness, thermal shock resistance, and chemical stability, making them ideal for high-temperature laboratory applications. Stoneware crucibles are more cost-effective but less durable, with lower resistance to thermal shock and chemical corrosion.
Table of Comparison
| Property | Boron Carbide | Stoneware |
|---|---|---|
| Chemical Composition | B4C (Boron and Carbon) | Al2O3, SiO2, other clay minerals |
| Melting Point | 2763degC | 1200-1300degC |
| Thermal Shock Resistance | Excellent | Moderate |
| Hardness (Mohs) | 9.5 | 6-7 |
| Density | 2.52 g/cm3 | 2.4-2.6 g/cm3 |
| Chemical Resistance | Highly resistant to acids and alkalis | Resistant to most chemicals, less resistant than B4C |
| Application in Lab Crucibles | Used for high-temperature, corrosive environments | Suitable for general-purpose heating up to moderate temperatures |
| Cost | High | Low to moderate |
Introduction to Laboratory Crucibles
Laboratory crucibles require materials that withstand extreme temperatures and chemical corrosion, making boron carbide and stoneware common choices. Boron carbide excels in thermal shock resistance and hardness, suitable for high-temperature applications exceeding 2000degC, while stoneware offers excellent chemical inertness and durability up to around 1400degC. Selecting between boron carbide and stoneware depends on specific lab conditions such as temperature ranges, chemical exposure, and mechanical stress.
Overview of Boron Carbide Crucibles
Boron carbide crucibles offer exceptional hardness, high thermal conductivity, and chemical inertness, making them ideal for high-temperature laboratory applications involving corrosive and abrasive materials. Their superior resistance to thermal shock and wear outperforms traditional stoneware crucibles, ensuring longer lifespan and consistent performance under extreme conditions. Boron carbide's low density and high melting point enable precise temperature control and efficient energy use during metal melting, ceramic sintering, and other high-temperature processes.
Overview of Stoneware Crucibles
Stoneware crucibles, made from refined clay fired at high temperatures, offer excellent thermal shock resistance and chemical durability for routine laboratory applications. They are cost-effective and suitable for heating non-corrosive substances up to approximately 1300degC. Although less resistant to abrasion and extreme chemical attack than boron carbide, stoneware crucibles maintain stability and integrity in general analytical processes.
Chemical Resistance Comparison
Boron carbide crucibles exhibit exceptional chemical resistance against strong acids and alkalis, making them highly suitable for aggressive chemical reactions in laboratories. Stoneware crucibles offer good resistance to moderate chemical corrosion but may degrade over time when exposed to strong acids or molten metals. The superior chemical stability of boron carbide ensures minimal contamination and longer service life under harsh chemical conditions compared to conventional stoneware.
Thermal Stability and Heat Tolerance
Boron carbide crucibles exhibit exceptional thermal stability and heat tolerance, enduring temperatures up to 2700degC without deformation, making them ideal for extreme high-temperature applications. Stoneware crucibles typically withstand temperatures around 1200degC to 1400degC, offering moderate thermal stability suitable for less intense laboratory processes. The superior heat resistance of boron carbide ensures enhanced durability and performance in environments requiring sustained exposure to ultra-high temperatures.
Mechanical Strength and Durability
Boron carbide crucibles exhibit superior mechanical strength compared to stoneware, with a hardness rating of about 9.5 on the Mohs scale, making them highly resistant to abrasion and impact. Their durability under extreme thermal shock and corrosive environments surpasses that of stoneware, which tends to be more brittle and susceptible to cracking under rapid temperature changes. Stoneware crucibles, while cost-effective and chemically inert, lack the robustness required for high-stress laboratory conditions where boron carbide provides longer service life and reliability.
Compatibility with Laboratory Processes
Boron carbide crucibles offer superior chemical resistance and thermal stability, making them ideal for high-temperature applications involving aggressive chemicals and molten metals. Stoneware crucibles exhibit excellent resistance to thermal shock and many acids but are prone to wear under extreme temperatures and reactive materials. Choosing between boron carbide and stoneware depends on specific laboratory processes, with boron carbide preferred for more demanding, corrosive environments and stoneware suited for general-purpose heating and less reactive substances.
Cost and Availability Analysis
Boron carbide crucibles offer exceptional thermal resistance and chemical stability but come at a significantly higher cost compared to stoneware, limiting their widespread use in budget-conscious laboratories. Stoneware crucibles are more readily available and economical, making them suitable for general-purpose applications where extreme conditions are not required. The choice between boron carbide and stoneware crucibles largely depends on the balance between performance requirements and budget constraints, with stoneware offering greater accessibility and lower upfront investment.
Maintenance and Lifespan Considerations
Boron carbide crucibles exhibit exceptional durability and high resistance to thermal shock, resulting in lower maintenance and an extended lifespan compared to stoneware crucibles. Stoneware crucibles, while cost-effective, are more prone to chipping and cracking under intense thermal cycling, leading to more frequent replacement and higher maintenance needs. The superior hardness and chemical inertness of boron carbide reduce contamination risks and downtime in laboratory applications, making it a preferred choice for long-term use.
Choosing the Right Crucible for Laboratory Applications
Boron carbide crucibles offer exceptional hardness, thermal shock resistance, and chemical inertness, making them ideal for high-temperature and aggressive chemical applications in laboratory settings. Stoneware crucibles provide cost-effective durability and good resistance to thermal shock and corrosion, suitable for routine lab use involving moderate temperatures and less aggressive materials. Selecting the right laboratory crucible depends on the specific thermal requirements, chemical compatibility, and budget constraints of the intended experimental procedures.
Infographic: Boron carbide vs Stoneware for Laboratory crucible
 azmater.com
azmater.com