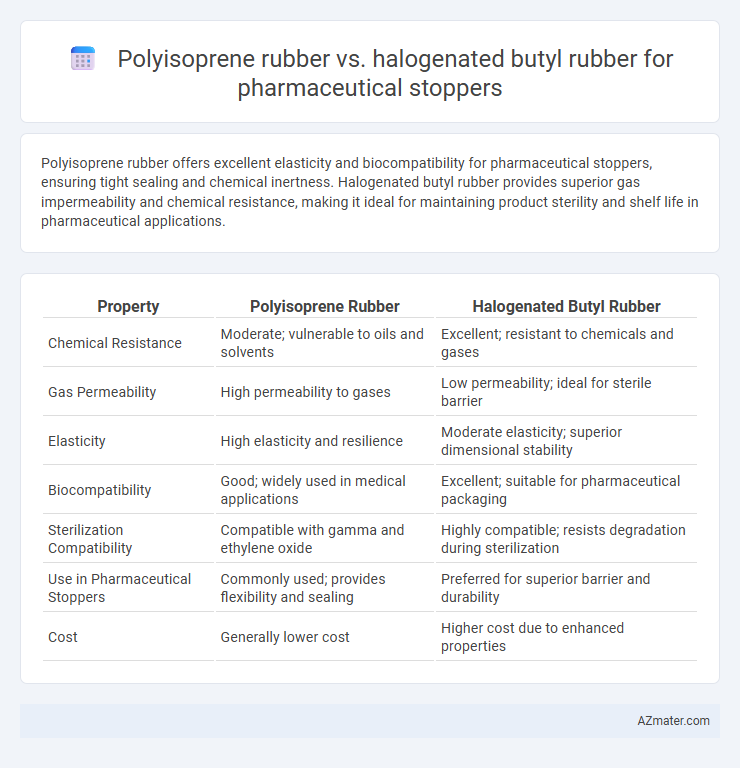
Polyisoprene rubber offers excellent elasticity and biocompatibility for pharmaceutical stoppers, ensuring tight sealing and chemical inertness. Halogenated butyl rubber provides superior gas impermeability and chemical resistance, making it ideal for maintaining product sterility and shelf life in pharmaceutical applications.
Table of Comparison
| Property | Polyisoprene Rubber | Halogenated Butyl Rubber |
|---|---|---|
| Chemical Resistance | Moderate; vulnerable to oils and solvents | Excellent; resistant to chemicals and gases |
| Gas Permeability | High permeability to gases | Low permeability; ideal for sterile barrier |
| Elasticity | High elasticity and resilience | Moderate elasticity; superior dimensional stability |
| Biocompatibility | Good; widely used in medical applications | Excellent; suitable for pharmaceutical packaging |
| Sterilization Compatibility | Compatible with gamma and ethylene oxide | Highly compatible; resists degradation during sterilization |
| Use in Pharmaceutical Stoppers | Commonly used; provides flexibility and sealing | Preferred for superior barrier and durability |
| Cost | Generally lower cost | Higher cost due to enhanced properties |
Introduction to Pharmaceutical Stoppers
Pharmaceutical stoppers require materials with exceptional chemical resistance, low permeability, and biocompatibility to ensure drug safety and efficacy. Polyisoprene rubber offers excellent flexibility and clarity but has moderate chemical resistance, while halogenated butyl rubber provides superior impermeability and enhanced resistance to sterilization processes, making it ideal for preserving drug integrity. Selecting the appropriate rubber type directly impacts the stopper's ability to maintain sterility and prevent contamination in pharmaceutical packaging.
Overview of Polyisoprene Rubber
Polyisoprene rubber is a synthetic elastomer closely resembling natural rubber, prized for its excellent elasticity, tensile strength, and chemical resistance, making it suitable for pharmaceutical stoppers requiring flexibility and durability. Its high purity and low extractables profile ensure compatibility with sensitive pharmaceutical formulations, reducing contamination risks during drug storage. Compared to halogenated butyl rubber, polyisoprene provides superior resilience and sealing performance under dynamic conditions, crucial for maintaining drug integrity.
Overview of Halogenated Butyl Rubber
Halogenated butyl rubber is a modified form of butyl rubber incorporating chlorine or bromine atoms to enhance chemical resistance and gas impermeability, making it ideal for pharmaceutical stoppers requiring high barrier properties. Compared to polyisoprene rubber, halogenated butyl offers superior resistance to sterilization processes, improved durability, and reduced extractables, crucial for maintaining drug stability and safety. Its low permeability to oxygen and moisture ensures extended shelf life, meeting stringent pharmaceutical packaging standards such as USP and EP.
Key Material Properties Comparison
Polyisoprene rubber offers excellent elasticity and high tensile strength, ensuring superior sealing performance and resilience against repeated use in pharmaceutical stoppers. Halogenated butyl rubber exhibits outstanding chemical resistance and low permeability to gases and moisture, critical for maintaining drug stability and shelf life. While polyisoprene is favored for its natural rubber-like properties, halogenated butyl's enhanced impermeability and resistance to sterilization chemicals make it a preferred choice for injectable drug containers.
Chemical Compatibility with Pharmaceuticals
Polyisoprene rubber exhibits excellent chemical compatibility with a wide range of pharmaceuticals due to its natural elastomeric properties and resistance to aqueous solutions, making it suitable for injectable drugs and vaccines. Halogenated butyl rubber offers superior resistance to aggressive chemicals, solvents, and sterilization processes, ensuring minimal interaction and contamination risk with pharmaceutical formulations. Selection depends on the specific drug composition, sterilization method, and regulatory compliance for ensuring stopper integrity and drug safety.
Gas and Moisture Permeability
Polyisoprene rubber exhibits higher gas and moisture permeability compared to halogenated butyl rubber, making it less effective in maintaining the sterility of pharmaceutical stoppers. Halogenated butyl rubber, particularly chlorobutyl and bromobutyl variants, offers superior barrier properties against oxygen, nitrogen, and moisture vapor transmission, ensuring enhanced chemical stability and shelf life of injectable drugs. The low permeability of halogenated butyl rubber minimizes gas ingress and moisture loss, crucial for preserving drug efficacy in parenteral packaging systems.
Sterilization Capabilities and Resistance
Polyisoprene rubber offers excellent sterilization capabilities and maintains its elasticity and integrity under gamma and autoclave sterilization, making it suitable for pharmaceutical stoppers requiring high biocompatibility. Halogenated butyl rubber demonstrates superior chemical resistance and impermeability, particularly against sterilizing agents and gases like ethylene oxide, ensuring long-term stability and contamination prevention. The choice between these materials hinges on the specific sterilization method and barrier properties needed for the pharmaceutical application.
Extractables and Leachables Concerns
Polyisoprene rubber exhibits lower extractables and leachables profiles compared to halogenated butyl rubber, making it more suitable for pharmaceutical stoppers where minimizing contamination risk is critical. Halogenated butyl rubber contains reactive halogen groups that can increase leachable impurities, potentially compromising drug stability and patient safety. Regulatory guidelines emphasize the importance of selecting stopper materials with minimal leachable substances, and polyisoprene's natural polymer matrix typically shows better compatibility with stringent pharmaceutical requirements.
Regulatory Compliance and Standards
Polyisoprene rubber and halogenated butyl rubber both meet stringent regulatory compliance requirements for pharmaceutical stoppers, adhering to USP <381> and ISO 8873 standards. Halogenated butyl rubber offers superior barrier properties against gases and moisture, enhancing drug product stability and meeting FDA and EMA packaging regulations. Polyisoprene rubber provides excellent elastomeric properties while complying with USP <661> and EP standards for injectable drug closures, ensuring safety and biocompatibility in pharmaceutical applications.
Application Suitability and Cost Considerations
Polyisoprene rubber offers excellent elasticity and low extractables, making it highly suitable for pharmaceutical stoppers requiring superior sealing and biocompatibility in injectable drug packaging. Halogenated butyl rubber provides enhanced chemical resistance and low permeability to gases, ideal for preserving the sterility and stability of sensitive pharmaceutical products. Cost considerations favor polyisoprene in budget-sensitive applications due to its lower raw material expenses, whereas halogenated butyl commands higher prices justified by extended shelf-life and barrier properties.
Infographic: Polyisoprene rubber vs Halogenated butyl rubber for Pharmaceutical stopper
 azmater.com
azmater.com