Polymethyl methacrylate (PMMA) offers excellent optical clarity and biocompatibility, making it suitable for ocular implants, while Polyether ether ketone (PEEK) provides superior mechanical strength, chemical resistance, and radiolucency, ideal for load-bearing orthopedic implants. PEEK's versatility in 3D printing and long-term stability enhances its application in complex, durable medical implants compared to the relatively brittle and less durable PMMA.
Table of Comparison
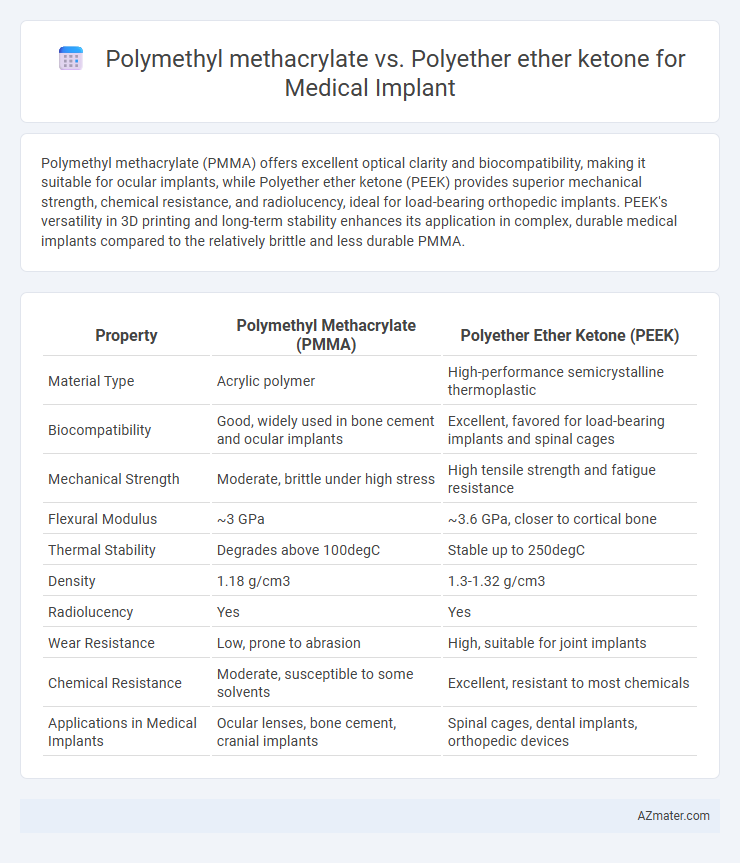
| Property | Polymethyl Methacrylate (PMMA) | Polyether Ether Ketone (PEEK) |
|---|---|---|
| Material Type | Acrylic polymer | High-performance semicrystalline thermoplastic |
| Biocompatibility | Good, widely used in bone cement and ocular implants | Excellent, favored for load-bearing implants and spinal cages |
| Mechanical Strength | Moderate, brittle under high stress | High tensile strength and fatigue resistance |
| Flexural Modulus | ~3 GPa | ~3.6 GPa, closer to cortical bone |
| Thermal Stability | Degrades above 100degC | Stable up to 250degC |
| Density | 1.18 g/cm3 | 1.3-1.32 g/cm3 |
| Radiolucency | Yes | Yes |
| Wear Resistance | Low, prone to abrasion | High, suitable for joint implants |
| Chemical Resistance | Moderate, susceptible to some solvents | Excellent, resistant to most chemicals |
| Applications in Medical Implants | Ocular lenses, bone cement, cranial implants | Spinal cages, dental implants, orthopedic devices |
Introduction to Medical Implant Materials
Polymethyl methacrylate (PMMA) and Polyether ether ketone (PEEK) are prominent materials in medical implant applications due to their biocompatibility and mechanical properties. PMMA offers excellent optical clarity and ease of fabrication, making it suitable for bone cement and intraocular lenses, while PEEK provides superior chemical resistance, high strength-to-weight ratio, and radiolucency, ideal for spinal implants and orthopedic devices. Both materials meet essential regulatory standards and demonstrate long-term stability within the human body, influencing their selection based on implant requirements and anatomical considerations.
Overview of Polymethyl Methacrylate (PMMA)
Polymethyl methacrylate (PMMA) is a transparent thermoplastic widely used in medical implants due to its excellent biocompatibility, high strength, and ease of molding. Its applications in orthopedic and cranial implants leverage PMMA's superior dimensional stability and resistance to biodegradation. Compared to polyether ether ketone (PEEK), PMMA offers cost-effective manufacturing and a well-established clinical track record but has lower toughness and fatigue resistance.
Properties of Polyether Ether Ketone (PEEK)
Polyether ether ketone (PEEK) exhibits exceptional mechanical strength, chemical resistance, and biocompatibility, making it highly suitable for medical implants compared to polymethyl methacrylate (PMMA). PEEK's low modulus of elasticity closely matches that of human bone, reducing stress shielding and promoting better osseointegration. Its radiolucency and thermal stability further enhance implant performance, especially in load-bearing orthopedic and spinal applications.
Mechanical Strength Comparison: PMMA vs PEEK
Polyether ether ketone (PEEK) exhibits superior mechanical strength compared to polymethyl methacrylate (PMMA), with higher tensile strength and improved fatigue resistance critical for load-bearing medical implants. PEEK's modulus of elasticity closely matches human cortical bone, enhancing implant integration and reducing stress shielding, whereas PMMA's lower mechanical properties limit its use primarily to bone cement and non-load-bearing applications. The durability and biocompatibility of PEEK make it a preferred choice for spinal, orthopedic, and dental implants where mechanical strength and longevity are essential.
Biocompatibility and Tissue Response
Polymethyl methacrylate (PMMA) exhibits excellent biocompatibility with minimal inflammatory response, making it suitable for bone cement and cranial implants; however, it may elicit fibrous encapsulation over time. Polyether ether ketone (PEEK) demonstrates superior tissue integration and bioinertness, promoting minimal immune reaction and stable long-term implantation in orthopedic and spinal devices. Comparative studies reveal PEEK's enhanced resistance to wear and lower cytotoxicity, favoring improved tissue response and implant longevity in medical applications.
Long-Term Durability and Wear Resistance
Polymethyl methacrylate (PMMA) offers good biocompatibility and optical clarity but exhibits lower long-term durability and wear resistance compared to Polyether ether ketone (PEEK), which demonstrates exceptional mechanical strength and chemical stability under prolonged physiological conditions. PEEK's high resistance to wear and fatigue makes it more suitable for load-bearing medical implants such as spinal cages and joint replacements, where longevity and minimal wear debris are critical. In contrast, PMMA is more commonly used in applications like bone cement or intraocular lenses where lower mechanical demands exist.
Radiolucency and Imaging Compatibility
Polymethyl methacrylate (PMMA) exhibits superior radiolucency compared to polyether ether ketone (PEEK), making it highly suitable for medical implants requiring clear X-ray and CT imaging without artifact interference. PEEK, while biocompatible and mechanically robust, tends to cause slight imaging artifacts due to its semi-crystalline structure, reducing diagnostic clarity in radiographic evaluations. Radiolucent PMMA implants facilitate enhanced post-operative monitoring and accurate imaging interpretation critical in orthopedic and dental applications.
Clinical Applications in Orthopedics and Dentistry
Polymethyl methacrylate (PMMA) is extensively used in orthopedic bone cement and dental prosthetics due to its biocompatibility, ease of molding, and strong adhesive properties. Polyether ether ketone (PEEK) is favored for spinal implants and dental implants owing to its superior mechanical strength, radiolucency, and excellent chemical resistance, which enhance osseointegration and long-term stability. Clinical studies highlight PMMA's rapid polymerization suited for bone fixation, while PEEK's durability and elastic modulus closely match cortical bone, reducing stress shielding in load-bearing orthopedic and dental applications.
Cost and Manufacturing Considerations
Polymethyl methacrylate (PMMA) offers a cost-effective solution for medical implants due to its lower material and processing expenses compared to polyether ether ketone (PEEK), which is significantly more expensive because of its complex synthesis and high-performance properties. Manufacturing PMMA implants involves simpler techniques such as molding and machining, facilitating faster production cycles and reduced labor costs, whereas PEEK requires advanced manufacturing methods like precision CNC machining and injection molding under stringent thermal controls, increasing overall costs. The choice between PMMA and PEEK balances budget constraints against mechanical performance, with PMMA favored for economic scalability and PEEK preferred for demanding biomechanical applications.
Future Trends and Innovations in Implant Materials
Polymethyl methacrylate (PMMA) and Polyether ether ketone (PEEK) are prominent materials in medical implants, with PMMA favored for its ease of molding and cost-effectiveness, while PEEK offers superior mechanical strength and biocompatibility. Future trends in implant materials emphasize enhancing bioactivity through surface modifications and incorporating nanotechnology to promote tissue integration and reduce infection risks. Innovations are also targeting the development of composite materials combining PMMA's versatility with PEEK's durability to create implants with tailored mechanical properties and improved patient outcomes.
Infographic: Polymethyl methacrylate vs Polyether ether ketone for Medical Implant
 azmater.com
azmater.com