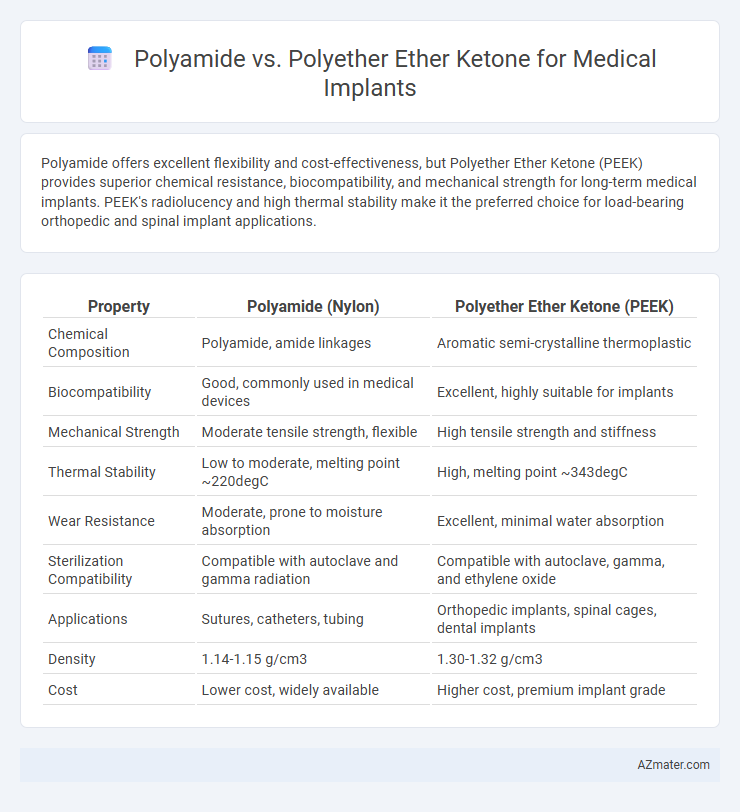
Polyamide offers excellent flexibility and cost-effectiveness, but Polyether Ether Ketone (PEEK) provides superior chemical resistance, biocompatibility, and mechanical strength for long-term medical implants. PEEK's radiolucency and high thermal stability make it the preferred choice for load-bearing orthopedic and spinal implant applications.
Table of Comparison
| Property | Polyamide (Nylon) | Polyether Ether Ketone (PEEK) |
|---|---|---|
| Chemical Composition | Polyamide, amide linkages | Aromatic semi-crystalline thermoplastic |
| Biocompatibility | Good, commonly used in medical devices | Excellent, highly suitable for implants |
| Mechanical Strength | Moderate tensile strength, flexible | High tensile strength and stiffness |
| Thermal Stability | Low to moderate, melting point ~220degC | High, melting point ~343degC |
| Wear Resistance | Moderate, prone to moisture absorption | Excellent, minimal water absorption |
| Sterilization Compatibility | Compatible with autoclave and gamma radiation | Compatible with autoclave, gamma, and ethylene oxide |
| Applications | Sutures, catheters, tubing | Orthopedic implants, spinal cages, dental implants |
| Density | 1.14-1.15 g/cm3 | 1.30-1.32 g/cm3 |
| Cost | Lower cost, widely available | Higher cost, premium implant grade |
Introduction to Polyamide and Polyether Ether Ketone in Medical Implants
Polyamide, known for its excellent mechanical strength and biocompatibility, is commonly used in medical implants requiring flexibility and durability, such as sutures and catheter components. Polyether Ether Ketone (PEEK) offers superior chemical resistance, high tensile strength, and radiolucency, making it ideal for load-bearing orthopedic implants and spinal devices. Both materials provide distinct advantages in medical applications, with polyamide favored for its adaptability and PEEK prized for its long-term stability and performance under physiological conditions.
Material Composition and Structure Comparison
Polyamide, commonly known as nylon, consists of repeating amide linkages (-CONH-) providing flexibility and moderate chemical resistance, while Polyether Ether Ketone (PEEK) features a semi-crystalline polymer chain with alternating ether and ketone groups that confer exceptional thermal stability and mechanical strength. The polyamide's molecular structure allows for higher moisture absorption, potentially affecting dimensional stability in implants, whereas PEEK's rigid aromatic backbone results in minimal water uptake and superior fatigue resistance. These structural distinctions make polyamide suitable for applications requiring elasticity and cost-efficiency, whereas PEEK's composition ensures long-term durability and biocompatibility for high-performance medical implants.
Mechanical Properties: Strength and Durability
Polyether ether ketone (PEEK) exhibits superior mechanical strength and exceptional durability compared to polyamide, making it highly suitable for long-term medical implants subjected to cyclic loading and stress. PEEK's high tensile strength, resistance to wear, and stable mechanical performance under sterilization processes exceed those of polyamide, which tends to absorb moisture and lose strength over time. The enhanced fatigue resistance and chemical stability of PEEK contribute significantly to implant longevity and biocompatibility, outperforming polyamide in critical orthopedic and dental applications.
Biocompatibility and Safety Considerations
Polyamide exhibits moderate biocompatibility with potential risks of inflammatory response and biofilm formation in long-term medical implants, while Polyether Ether Ketone (PEEK) offers superior biocompatibility due to its inert chemical structure, minimizing immune reactions and enhancing patient safety. PEEK's resistance to sterilization processes and chemical degradation further supports its use in medical implants requiring durability and reliability. Safety considerations emphasize PEEK's lower cytotoxicity and reduced risk of implant-associated infections compared to polyamide, making it a preferred material for critical biomedical applications.
Chemical Resistance and Sterilization Requirements
Polyether ether ketone (PEEK) demonstrates superior chemical resistance compared to polyamide, resisting degradation from a broad spectrum of solvents, acids, and alkalis commonly encountered in medical environments. PEEK withstands high-temperature sterilization methods such as autoclaving at 134degC, gamma radiation, and ethylene oxide without compromising mechanical integrity or biocompatibility, whereas polyamide is more susceptible to hydrolysis and may degrade under repeated sterilization cycles. These properties make PEEK a preferred material for long-term implantable devices requiring rigorous sterilization and chemical stability.
Wear Resistance and Longevity in Implant Applications
Polyether Ether Ketone (PEEK) exhibits superior wear resistance compared to Polyamide, making it more suitable for long-term medical implant applications where durability is critical. The high mechanical strength and chemical stability of PEEK contribute to its enhanced longevity, minimizing implant degradation over time under physiological conditions. In contrast, Polyamide, while cost-effective and biocompatible, tends to have lower abrasion resistance and can absorb moisture, which may reduce its lifespan in demanding implant environments.
Processing Techniques: Molding and Machining
Polyamide offers ease of processing through injection molding and precision machining, making it suitable for complex, customized medical implant designs with moderate thermal resistance. Polyether ether ketone (PEEK) requires higher processing temperatures during injection molding but provides superior mechanical strength and chemical resistance, making it ideal for implants subjected to high stress and sterilization cycles. Machining PEEK demands specialized tooling due to its hardness, whereas polyamide's machinability supports cost-effective prototyping and rapid production.
Cost Analysis and Market Availability
Polyamide offers a lower-cost solution for medical implants, benefiting from widespread market availability and established manufacturing processes, making it accessible for a broad range of applications. Polyether Ether Ketone (PEEK), while significantly more expensive, provides superior mechanical properties and chemical resistance, positioning it as a premium choice for high-performance implants. Market analysis shows polyamide dominates in cost-sensitive segments, whereas PEEK commands a niche in specialized, high-demand medical implant markets due to its enhanced biocompatibility and durability.
Clinical Applications: Case Studies and Performance
Polyether Ether Ketone (PEEK) outperforms polyamide in medical implants due to superior biocompatibility, chemical resistance, and mechanical strength, which are critical in load-bearing orthopedic applications and spinal cages. Clinical case studies demonstrate PEEK's ability to integrate well with bone tissue, reducing inflammation and long-term implant failure rates compared to polyamide, which often exhibits higher wear and susceptibility to hydrolysis. PEEK's stable performance in sterilization processes and MRI compatibility further establishes it as the preferred material for durable, high-performance medical implants in demanding clinical environments.
Conclusion: Choosing the Ideal Polymer for Medical Implants
Polyether Ether Ketone (PEEK) offers superior biocompatibility, chemical resistance, and mechanical strength compared to polyamide, making it the preferred choice for long-term medical implants. Polyamide, while cost-effective and easier to process, may suffer from moisture absorption and inferior durability under physiological conditions. Selecting PEEK ensures enhanced implant longevity and patient safety in demanding medical applications.
Infographic: Polyamide vs Polyether Ether Ketone for Medical Implant
 azmater.com
azmater.com