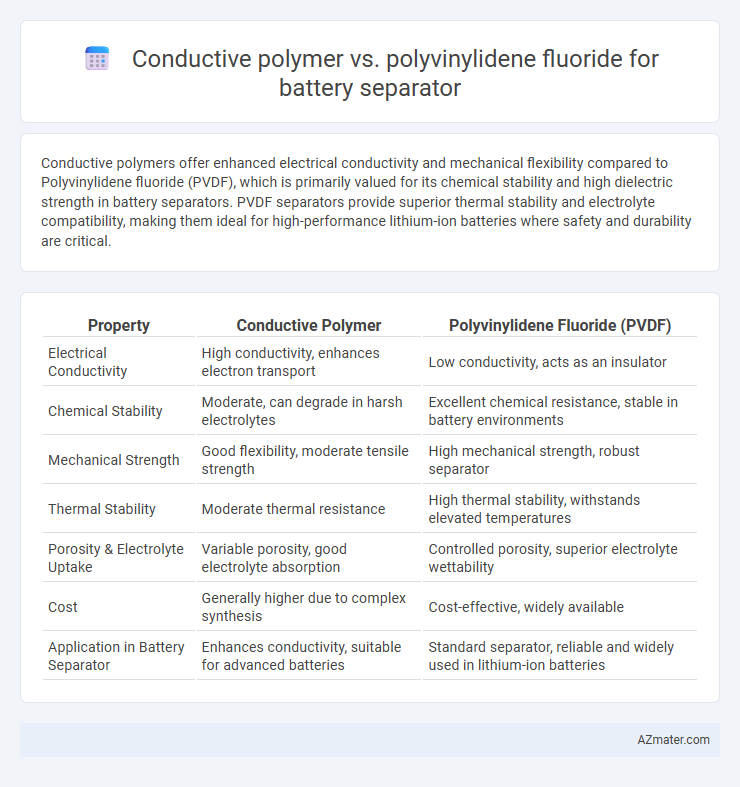
Conductive polymers offer enhanced electrical conductivity and mechanical flexibility compared to Polyvinylidene fluoride (PVDF), which is primarily valued for its chemical stability and high dielectric strength in battery separators. PVDF separators provide superior thermal stability and electrolyte compatibility, making them ideal for high-performance lithium-ion batteries where safety and durability are critical.
Table of Comparison
| Property | Conductive Polymer | Polyvinylidene Fluoride (PVDF) |
|---|---|---|
| Electrical Conductivity | High conductivity, enhances electron transport | Low conductivity, acts as an insulator |
| Chemical Stability | Moderate, can degrade in harsh electrolytes | Excellent chemical resistance, stable in battery environments |
| Mechanical Strength | Good flexibility, moderate tensile strength | High mechanical strength, robust separator |
| Thermal Stability | Moderate thermal resistance | High thermal stability, withstands elevated temperatures |
| Porosity & Electrolyte Uptake | Variable porosity, good electrolyte absorption | Controlled porosity, superior electrolyte wettability |
| Cost | Generally higher due to complex synthesis | Cost-effective, widely available |
| Application in Battery Separator | Enhances conductivity, suitable for advanced batteries | Standard separator, reliable and widely used in lithium-ion batteries |
Introduction to Battery Separator Materials
Battery separators play a crucial role in lithium-ion batteries by preventing physical contact between the anode and cathode while allowing ionic transport. Conductive polymers offer enhanced electrical conductivity and flexibility, making them promising candidates for improving separator performance with better mechanical strength and thermal stability. Polyvinylidene fluoride (PVDF) remains widely used due to its excellent chemical resistance, electrochemical stability, and strong mechanical properties, ensuring reliable ion permeability and safety in battery operation.
Overview of Conductive Polymers
Conductive polymers, such as polyaniline and polypyrrole, offer unique electrical conductivity combined with mechanical flexibility, making them promising materials for battery separators compared to traditional insulators like polyvinylidene fluoride (PVDF). These polymers facilitate improved ion transport and enhanced electrochemical stability, addressing key limitations in battery performance and safety. Their tunable properties enable customized separator functionality, potentially increasing battery efficiency and lifespan.
Polyvinylidene Fluoride (PVDF): Key Features
Polyvinylidene fluoride (PVDF) offers excellent chemical stability, high thermal resistance, and superior mechanical strength, making it an ideal material for battery separators. Its inherent electrochemical stability and good electrolyte wettability enhance ionic conductivity and battery safety during charge-discharge cycles. Compared to conductive polymers, PVDF provides enhanced durability and consistent performance under high voltage and temperature conditions in lithium-ion batteries.
Ionic Conductivity and Electrochemical Performance
Conductive polymers exhibit enhanced ionic conductivity compared to polyvinylidene fluoride (PVDF), enabling more efficient ion transport in battery separators. PVDF offers excellent electrochemical stability and mechanical strength but generally has lower ionic conductivity due to its non-conductive nature. The superior electrochemical performance of conductive polymer-based separators results in improved charge-discharge rates and cycling stability in lithium-ion batteries.
Mechanical Strength and Durability Comparison
Conductive polymers generally exhibit superior mechanical strength and enhanced durability compared to polyvinylidene fluoride (PVDF) when used as battery separators, owing to their inherent flexibility and resistance to mechanical stress. PVDF, while chemically stable and possessing good thermal resistance, tends to have lower tensile strength and is more prone to mechanical degradation under prolonged cycling conditions. The robust mechanical integrity of conductive polymers improves the separator's lifespan and safety in high-performance battery applications.
Thermal Stability and Safety Aspects
Conductive polymers demonstrate superior thermal stability in battery separators due to their ability to maintain mechanical integrity at elevated temperatures, reducing the risk of thermal runaway. Polyvinylidene fluoride (PVDF), while offering excellent chemical resistance and mechanical strength, has a relatively lower thermal decomposition temperature, which may compromise safety under extreme thermal conditions. The inherent conductivity of conductive polymers also enhances thermal management and safety by facilitating uniform heat dissipation across the separator.
Chemical Compatibility with Battery Electrolytes
Conductive polymers exhibit excellent chemical compatibility with battery electrolytes due to their stable conjugated backbone and resistance to electrolyte decomposition, which enhances overall battery performance. Polyvinylidene fluoride (PVDF) is highly compatible with commonly used lithium-ion battery electrolytes, offering superior chemical resistance and electrolyte wettability, essential for efficient ionic conductivity. While conductive polymers provide functional advantages such as electrical conductivity, PVDF remains the preferred separator material because of its proven chemical stability and inertness in aggressive electrolyte environments.
Manufacturing Processes and Scalability
Conductive polymers like polyaniline offer enhanced electrical conductivity but involve complex synthesis processes such as chemical or electrochemical polymerization, which can limit large-scale production. Polyvinylidene fluoride (PVDF), known for its chemical stability and mechanical strength, is manufactured through well-established processes like solution casting and extrusion, enabling cost-effective scalability for battery separator applications. The comparatively simpler processing and robust scalability of PVDF make it more suitable for commercial battery separator manufacturing than conductive polymers.
Cost Analysis and Commercial Viability
Conductive polymers generally have higher material and processing costs compared to polyvinylidene fluoride (PVDF), which remains a cost-effective choice for battery separators due to its established manufacturing infrastructure and scalability. PVDF offers excellent chemical stability, mechanical strength, and electrolyte compatibility at a lower price point, enhancing its commercial viability in large-scale lithium-ion battery production. While conductive polymers enable improved electrical conductivity, their cost-intensive synthesis and limited large-scale production hinder widespread adoption in cost-sensitive battery markets.
Future Trends and Research Directions
Conductive polymers and polyvinylidene fluoride (PVDF) are pivotal materials in battery separators, with future trends emphasizing enhancing ionic conductivity and mechanical stability through nanocomposite and hybrid designs. Research is increasingly directed toward integrating conductive polymers with PVDF to leverage their combined electrochemical properties, enabling higher energy density and improved thermal safety in lithium-ion and solid-state batteries. Innovations in molecular doping, surface modification, and 3D structuring are expected to advance separator performance, facilitating next-generation energy storage devices with longer lifespans and faster charging capabilities.
Infographic: Conductive polymer vs Polyvinylidene fluoride for Battery separator
 azmater.com
azmater.com