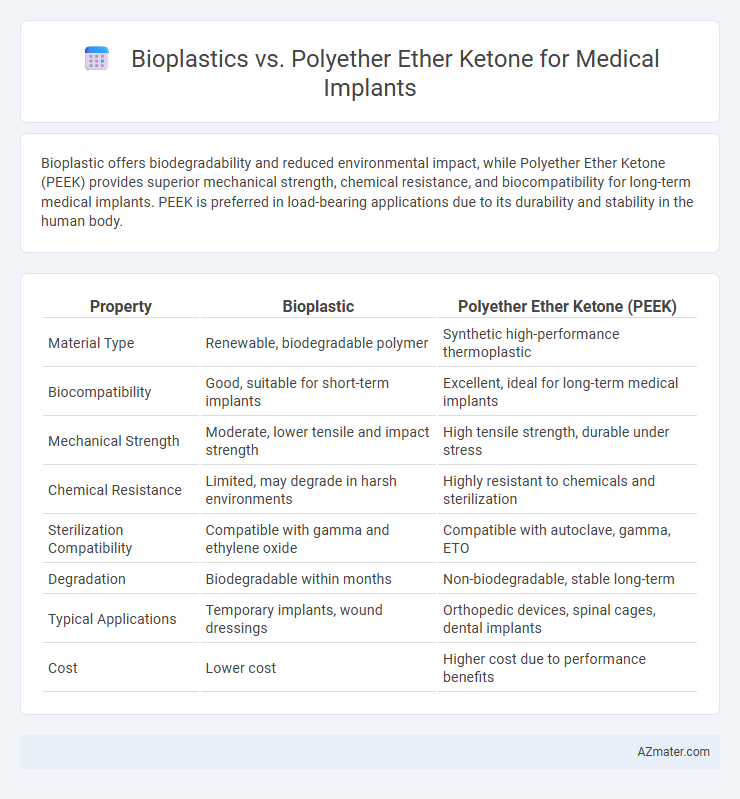
Bioplastic offers biodegradability and reduced environmental impact, while Polyether Ether Ketone (PEEK) provides superior mechanical strength, chemical resistance, and biocompatibility for long-term medical implants. PEEK is preferred in load-bearing applications due to its durability and stability in the human body.
Table of Comparison
| Property | Bioplastic | Polyether Ether Ketone (PEEK) |
|---|---|---|
| Material Type | Renewable, biodegradable polymer | Synthetic high-performance thermoplastic |
| Biocompatibility | Good, suitable for short-term implants | Excellent, ideal for long-term medical implants |
| Mechanical Strength | Moderate, lower tensile and impact strength | High tensile strength, durable under stress |
| Chemical Resistance | Limited, may degrade in harsh environments | Highly resistant to chemicals and sterilization |
| Sterilization Compatibility | Compatible with gamma and ethylene oxide | Compatible with autoclave, gamma, ETO |
| Degradation | Biodegradable within months | Non-biodegradable, stable long-term |
| Typical Applications | Temporary implants, wound dressings | Orthopedic devices, spinal cages, dental implants |
| Cost | Lower cost | Higher cost due to performance benefits |
Introduction to Medical Implant Materials
Medical implant materials require biocompatibility, mechanical strength, and durability to ensure safety and effectiveness in the human body. Bioplastics offer eco-friendly, biodegradable options with moderate strength, suitable for temporary implants. Polyether ether ketone (PEEK) provides superior chemical resistance, high mechanical performance, and long-term stability, making it ideal for permanent orthopedic and dental implants.
Overview of Bioplastic in Medical Applications
Bioplastics in medical applications are gaining traction due to their biodegradability, biocompatibility, and reduced environmental impact compared to conventional polymers. These materials, derived from renewable resources like starch, polylactic acid (PLA), and polyhydroxyalkanoates (PHA), are increasingly used for temporary implants, sutures, and drug delivery systems. Their ability to degrade safely within the body and minimize long-term foreign material presence makes them a promising alternative to traditional high-performance materials like polyether ether ketone (PEEK) in certain medical implant scenarios.
Understanding Polyether Ether Ketone (PEEK)
Polyether Ether Ketone (PEEK) is a high-performance thermoplastic known for its exceptional mechanical strength, chemical resistance, and biocompatibility, making it ideal for medical implants such as spinal cages and dental devices. Unlike bioplastics, PEEK maintains stability under sterilization processes and exhibits superior wear resistance, ensuring long-term implant durability. Its radiolucency also allows clear imaging during postoperative evaluations, enhancing patient monitoring and treatment outcomes.
Biocompatibility: Bioplastic vs PEEK
Bioplastics exhibit excellent biocompatibility due to their natural origin and biodegradability, minimizing inflammatory responses in medical implants. Polyether Ether Ketone (PEEK) offers superior chemical resistance and mechanical stability while maintaining biocompatibility, making it ideal for load-bearing implants. Comparative studies highlight PEEK's enhanced suitability for long-term implantation due to its inertness and resistance to sterilization processes.
Mechanical Properties Comparison
Bioplastic materials used in medical implants offer moderate tensile strength and flexibility, making them suitable for applications requiring biodegradability and temporary support. Polyether Ether Ketone (PEEK) exhibits superior mechanical properties, including high tensile strength (up to 90-100 MPa), exceptional fatigue resistance, and a modulus of elasticity (~3.6 GPa) closely matching cortical bone, which enhances implant stability and load-bearing capacity. The chemical resistance and thermal stability of PEEK surpass most bioplastics, ensuring long-term performance in demanding physiological environments.
Sterilization and Chemical Resistance
Polyether Ether Ketone (PEEK) exhibits superior sterilization compatibility, tolerating autoclaving, gamma radiation, and ethylene oxide without compromising mechanical integrity, essential for medical implants requiring repeated sterilization. Bioplastics often degrade or deform under high-temperature steam sterilization and can be susceptible to chemical attack from common sterilants, limiting their use in harsh sterilization protocols. The exceptional chemical resistance of PEEK to acids, bases, and organic solvents ensures long-term implant stability, whereas bioplastics may undergo hydrolysis or oxidation, impacting implant safety and durability.
Longevity and Degradation Profiles
Polyether ether ketone (PEEK) exhibits exceptional longevity with thermal stability up to 250degC and hydrolytic resistance, making it ideal for long-term medical implants. Bioplastics, such as polylactic acid (PLA), demonstrate biodegradation within months to years under physiological conditions, offering temporary scaffold applications but limited durability. PEEK's inert nature resists enzymatic degradation, ensuring structural integrity over decades, while bioplastics degrade via hydrolytic and enzymatic pathways, leading to controlled resorption but shorter implant lifespan.
Regulatory Approvals and Certifications
Bioplastic and Polyether Ether Ketone (PEEK) differ significantly in regulatory approvals and certifications for medical implants, with PEEK widely recognized due to its established biocompatibility and proven FDA clearance for spinal, cranial, and dental applications. Bioplastics require extensive material-specific validation to meet ISO 10993 biological evaluation standards and FDA 21 CFR Part 820 quality system regulations, often facing variability challenges in consistency and sterilization benchmarks. The pharmaceutical-grade certifications and long-term implant data on PEEK provide a regulatory advantage, positioning it as a preferred material in implantable devices requiring rigorous safety and efficacy documentation.
Cost and Manufacturing Considerations
Bioplastics offer a cost-effective and sustainable alternative to Polyether Ether Ketone (PEEK) in medical implants, with lower raw material expenses and simpler manufacturing processes like injection molding. PEEK requires high-temperature processing and precise machining, leading to increased manufacturing costs but delivers superior mechanical strength and chemical resistance ideal for long-term implant durability. Evaluating production scalability, bioplastics allow faster prototyping and eco-friendly disposal, while PEEK demands specialized equipment and expertise, impacting overall cost-efficiency in medical device manufacturing.
Future Perspectives in Medical Implant Materials
Emerging advancements in bioplastics focus on enhanced biodegradability and biocompatibility, aiming to reduce long-term complications in medical implants. Polyether ether ketone (PEEK) remains a gold standard due to its exceptional mechanical strength, chemical resistance, and radiolucency for precise imaging. Future research prioritizes hybrid composites combining bioplastics with PEEK to optimize implant performance, promote tissue integration, and minimize environmental impact in medical applications.
Infographic: Bioplastic vs Polyether Ether Ketone for Medical Implant
 azmater.com
azmater.com