Vanadium batteries offer longer cycle life and better thermal stability compared to nickel-based batteries, making them ideal for large-scale energy storage. Nickel batteries provide higher energy density and faster charging rates, commonly used in electric vehicles and portable electronics.
Table of Comparison
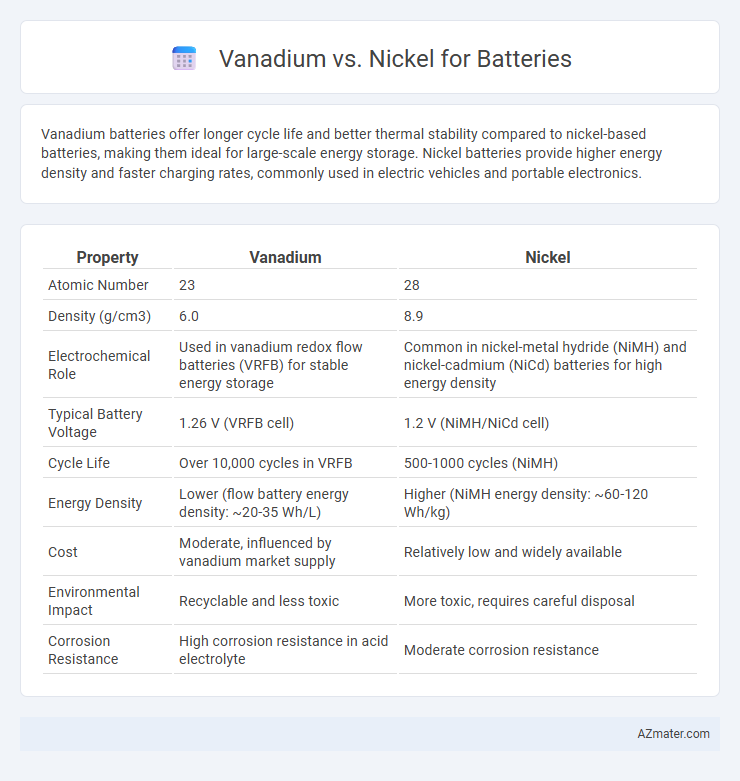
| Property | Vanadium | Nickel |
|---|---|---|
| Atomic Number | 23 | 28 |
| Density (g/cm3) | 6.0 | 8.9 |
| Electrochemical Role | Used in vanadium redox flow batteries (VRFB) for stable energy storage | Common in nickel-metal hydride (NiMH) and nickel-cadmium (NiCd) batteries for high energy density |
| Typical Battery Voltage | 1.26 V (VRFB cell) | 1.2 V (NiMH/NiCd cell) |
| Cycle Life | Over 10,000 cycles in VRFB | 500-1000 cycles (NiMH) |
| Energy Density | Lower (flow battery energy density: ~20-35 Wh/L) | Higher (NiMH energy density: ~60-120 Wh/kg) |
| Cost | Moderate, influenced by vanadium market supply | Relatively low and widely available |
| Environmental Impact | Recyclable and less toxic | More toxic, requires careful disposal |
| Corrosion Resistance | High corrosion resistance in acid electrolyte | Moderate corrosion resistance |
Introduction to Vanadium and Nickel in Batteries
Vanadium and nickel serve crucial roles in battery technologies due to their electrochemical properties and energy storage capabilities. Vanadium is predominantly used in vanadium redox flow batteries (VRFBs), offering high stability, scalability, and long cycle life, which makes it ideal for large-scale energy storage. Nickel, commonly found in nickel-metal hydride (NiMH) and nickel-cadmium (NiCd) batteries, provides high energy density and robust performance, making it suitable for portable electronics and electric vehicles.
Chemical Properties: Vanadium vs Nickel
Vanadium exhibits multiple stable oxidation states ranging from +2 to +5, enabling versatile redox reactions critical for high-capacity energy storage, while nickel primarily exists in +2 and +3 states, offering stable electrochemical behavior but limited redox flexibility. The ionic radius of vanadium ions allows for efficient intercalation in battery electrodes, enhancing charge-discharge cycles, whereas nickel's smaller ionic size contributes to higher conductivity but less structural adaptability. Vanadium's chemical stability in acidic electrolytes supports vanadium redox flow batteries, contrasting with nickel's better performance in alkaline environments typical of nickel-cadmium and nickel-metal hydride batteries.
Energy Density Comparison
Vanadium redox flow batteries typically offer lower energy density, around 20-40 Wh/L, compared to nickel-based batteries, which can reach energy densities of 150-250 Wh/kg. Nickel batteries, such as nickel-metal hydride (NiMH) or nickel-cadmium (NiCd), provide higher volumetric and gravimetric energy storage, making them more suitable for applications requiring compact and lightweight power sources. Vanadium batteries excel in scalability and cycle life but lag behind nickel systems in energy density, limiting their use in portable electronics.
Cost Analysis of Vanadium and Nickel
Vanadium and nickel prices significantly impact battery manufacturing costs, with nickel generally priced higher due to its widespread use in lithium-ion batteries, averaging around $20,000 per metric ton compared to vanadium's approximate $8,000 per metric ton. Vanadium redox flow batteries benefit from lower raw material costs and longer cycle life, reducing overall cost per kilowatt-hour despite a higher initial investment in electrolyte solutions. Fluctuations in global supply chains, particularly nickel's reliance on limited mining regions, further influence market prices and cost stability for battery producers.
Cycle Life and Durability
Vanadium-based batteries, particularly vanadium redox flow batteries (VRFBs), exhibit exceptional cycle life exceeding 10,000 cycles with minimal capacity degradation, making them highly durable for long-term energy storage. Nickel-based batteries, such as nickel-cadmium (NiCd) and nickel-metal hydride (NiMH), offer moderate cycle lives of around 500 to 1,000 cycles, with gradual decline in performance due to electrode degradation. The superior chemical stability and reversible redox reactions in vanadium systems significantly enhance durability and cycle life compared to nickel batteries.
Safety and Thermal Stability
Vanadium batteries exhibit superior thermal stability compared to nickel-based batteries, reducing risks of thermal runaway and enhancing overall safety in energy storage applications. Vanadium redox flow batteries operate at lower temperatures and have non-flammable electrolytes, minimizing hazards during thermal stress or mechanical failure. Nickel batteries, while offering higher energy density, face challenges with overheating and thermal decomposition, necessitating advanced thermal management systems for safe operation.
Environmental Impact and Sustainability
Vanadium batteries offer superior environmental sustainability due to their long cycle life and high recyclability, reducing waste and resource extraction compared to nickel-based batteries. Nickel extraction is associated with significant ecological degradation and toxic emissions, impacting biodiversity and local communities. Vanadium's stable and abundant supply chain supports greener energy storage solutions with lower carbon footprints in production and recycling processes.
Current Market Applications
Vanadium is primarily used in vanadium redox flow batteries, which are favored for large-scale energy storage due to their long cycle life and scalability, especially in renewable energy integration and grid stabilization. Nickel dominates lithium-ion battery cathodes, particularly in electric vehicles (EVs) and portable electronics, offering high energy density and power output. Current market trends show vanadium flow batteries addressing stationary storage solutions while nickel-based batteries lead in mobility and consumer electronics sectors.
Future Trends in Battery Materials
Vanadium-based flow batteries are gaining traction for large-scale energy storage due to their long cycle life and safety advantages over nickel-based batteries, which primarily dominate in electric vehicles. Emerging research highlights the potential of vanadium redox flow batteries to address grid stability with scalable capacity, while nickel continues to improve energy density and charging speed through advanced cathode chemistry. Future trends suggest a hybrid approach, leveraging vanadium's durability and nickel's power output to meet evolving energy storage demands efficiently.
Conclusion: Choosing Between Vanadium and Nickel
Vanadium offers superior thermal stability and long cycle life, making it ideal for large-scale energy storage, while nickel delivers higher energy density, favoring applications requiring compact and lightweight batteries. The choice depends on specific use cases: vanadium suits grid storage and renewable integration, whereas nickel is preferable for electric vehicles and portable electronics. Cost considerations and resource availability also influence the decision between vanadium and nickel-based batteries.
Infographic: Vanadium vs Nickel for Battery
 azmater.com
azmater.com