Osmium, a dense and stable transition metal with high corrosion resistance, is widely used in research requiring durable materials and precise instrumentation. Dubnium, a synthetic and highly radioactive element with a short half-life, is primarily studied for its nuclear properties and contributes to advancing our understanding of heavy element synthesis and nuclear chemistry.
Table of Comparison
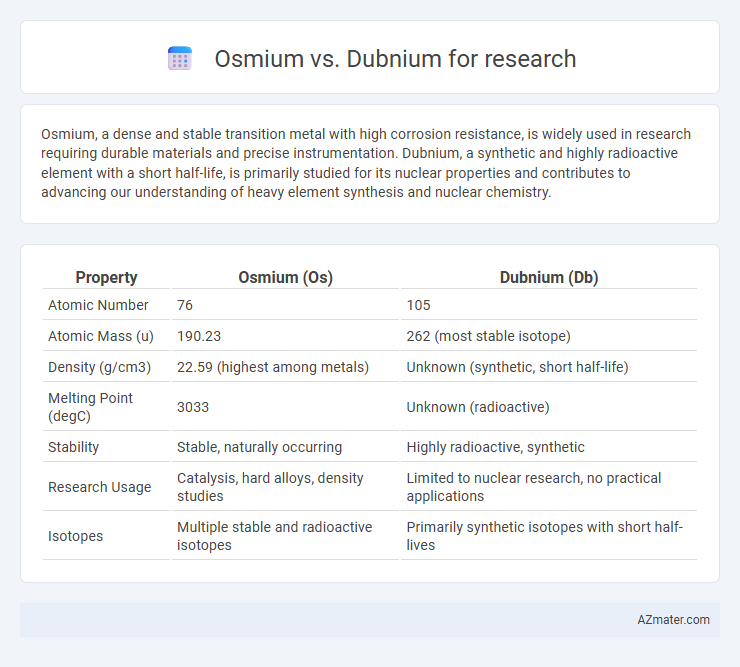
| Property | Osmium (Os) | Dubnium (Db) |
|---|---|---|
| Atomic Number | 76 | 105 |
| Atomic Mass (u) | 190.23 | 262 (most stable isotope) |
| Density (g/cm3) | 22.59 (highest among metals) | Unknown (synthetic, short half-life) |
| Melting Point (degC) | 3033 | Unknown (radioactive) |
| Stability | Stable, naturally occurring | Highly radioactive, synthetic |
| Research Usage | Catalysis, hard alloys, density studies | Limited to nuclear research, no practical applications |
| Isotopes | Multiple stable and radioactive isotopes | Primarily synthetic isotopes with short half-lives |
Introduction to Osmium and Dubnium
Osmium, atomic number 76, is a dense, hard transition metal known for its high melting point and significant applications in catalysis and electrically resistant alloys. Dubnium, atomic number 105, is a synthetic, highly radioactive element with no stable isotopes, studied primarily through particle accelerator experiments to explore its chemical properties and placement in the transactinide series. Research comparing osmium and dubnium highlights differences in natural occurrence, practical uses, and the challenges of investigating synthetic superheavy elements.
Historical Discovery and Naming
Osmium, discovered in 1803 by Smithson Tennant, is named after the Greek word "osme," meaning smell, reflecting its distinct odor from osmium tetroxide. Dubnium, identified in the 1960s, honors Dubna, Russia, where the Joint Institute for Nuclear Research conducted pioneering work on synthetic elements. Both elements represent significant milestones in chemical research, with osmium rooted in early transition metal studies and dubnium emerging from advancements in nuclear chemistry.
Atomic Structure and Physical Properties
Osmium (Os), atomic number 76, features a dense hexagonal close-packed crystal structure, making it one of the heaviest and most stable transition metals with a high melting point of 3045degC and exceptional hardness. Dubnium (Db), atomic number 105, is a synthetic, highly unstable element with a short half-life and unknown stable isotopes, limiting its detailed physical characterization; it is expected to exhibit properties similar to group 5 transition metals, potentially having a body-centered cubic structure. The atomic structure of Osmium allows extensive research on dense, durable materials, while Dubnium's fleeting existence primarily confines its study to nuclear reactions and theoretical computational models.
Availability and Natural Occurrence
Osmium, a dense transition metal found naturally in Earth's crust, is relatively rare but accessible through mining of platinum-group metals, making it available for scientific and industrial research. Dubnium, a synthetic element produced only in particle accelerators through nuclear reactions, does not occur naturally and is available solely in minute quantities for experimental studies. The stark contrast in availability and natural occurrence positions osmium as a practical material for research, whereas dubnium remains a rare subject of investigation limited to advanced nuclear chemistry laboratories.
Synthesis Methods and Laboratory Production
Osmium is synthesized through mining and refining its naturally occurring ore as one of the densest stable elements, primarily obtained from platinum ores with high-pressure extraction techniques in laboratories worldwide. Dubnium, an artificial transactinide element, is produced exclusively via particle accelerator synthesis through fusion-evaporation reactions involving lighter elements like Californium or Berkelium targets bombarded with Neon or Nitrogen ions. Laboratory production of Dubnium requires specialized facilities such as cyclotrons capable of precise ion beam control, while Osmium extraction relies on large-scale industrial metallurgical processes.
Chemical Reactivity and Stability
Osmium exhibits exceptional chemical stability due to its dense atomic structure and resistance to oxidation, making it favorable for catalytic and electrochemical research applications. Dubnium, a synthetic transactinide element with a short half-life and limited availability, demonstrates high chemical reactivity and instability, posing significant challenges for experimental studies. The stark contrast in chemical behavior between osmium's robustness and dubnium's transient existence limits dubnium's practical use compared to osmium's extensive research versatility.
Applications in Scientific Research
Osmium exhibits exceptional density and catalytic properties, making it valuable for applications in high-precision scientific instrumentation and catalysis studies. Dubnium, a synthetic element with a very short half-life, primarily serves as a subject for nuclear chemistry and atomic structure research rather than practical applications. Current scientific research leverages osmium's stability for experimental reproducibility, whereas dubnium's primary value lies in advancing theoretical models of transactinide elements.
Safety Precautions and Toxicity
Osmium, a dense transition metal, poses significant toxicity risks mainly due to its oxide form, osmium tetroxide, which is highly volatile and harmful to respiratory tissues, requiring stringent handling procedures such as working in fume hoods and using protective gloves. Dubnium, a synthetic highly radioactive element with no stable isotopes and extremely limited availability, demands robust radiological safety protocols including shielding, remote handling, and strict contamination controls to mitigate radiation exposure risks. Research involving both elements prioritizes rigorous safety measures, but dubnium's radiotoxicity and short half-life impose far greater challenges in containment and experimental handling compared to osmium's chemical toxicity risks.
Cost and Accessibility for Researchers
Osmium, one of the densest naturally occurring elements, is relatively accessible and affordable for researchers, with costs generally ranging from $400 to $700 per ounce due to its presence in platinum ores and established extraction methods. Dubnium, a synthetic and highly radioactive transactinide element, is extremely costly and difficult to obtain, as it can only be produced in minute quantities through particle accelerator collisions and has a half-life measured in seconds. Consequently, osmium offers practical advantages in cost and availability, making it a more feasible option for experimental research compared to dubnium's limited accessibility and prohibitive expenses.
Future Prospects in Elemental Research
Osmium, a dense transition metal with well-characterized properties, serves as a critical reference point in high-pressure and materials science research, while dubnium, a synthetic element with atomic number 105, remains largely unexplored due to its radioactivity and short half-life. Future prospects in elemental research emphasize developing advanced methods for synthesizing and stabilizing dubnium isotopes to unlock potential applications in nuclear science and chemistry. Innovations in particle accelerator technology and detection techniques are expected to enhance understanding of dubnium's chemical properties, enabling comparisons with osmium and expanding the periodic table's frontiers.
Infographic: Osmium vs Dubnium for Research
 azmater.com
azmater.com