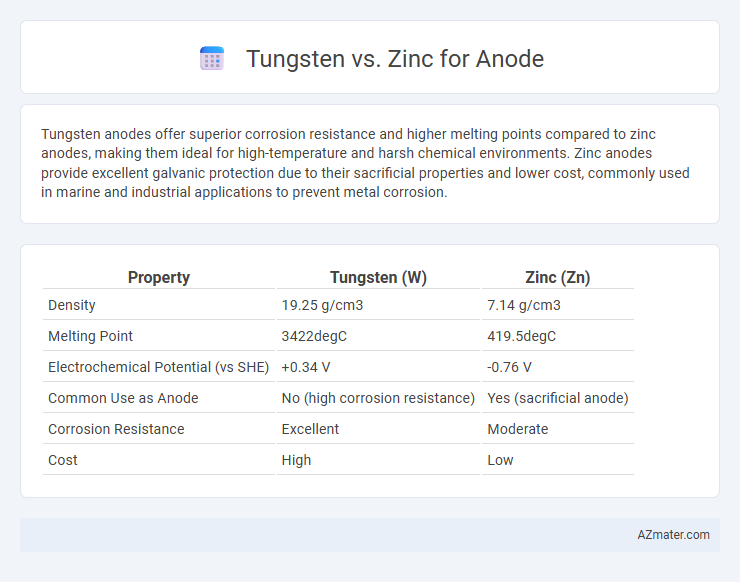
Tungsten anodes offer superior corrosion resistance and higher melting points compared to zinc anodes, making them ideal for high-temperature and harsh chemical environments. Zinc anodes provide excellent galvanic protection due to their sacrificial properties and lower cost, commonly used in marine and industrial applications to prevent metal corrosion.
Table of Comparison
| Property | Tungsten (W) | Zinc (Zn) |
|---|---|---|
| Density | 19.25 g/cm3 | 7.14 g/cm3 |
| Melting Point | 3422degC | 419.5degC |
| Electrochemical Potential (vs SHE) | +0.34 V | -0.76 V |
| Common Use as Anode | No (high corrosion resistance) | Yes (sacrificial anode) |
| Corrosion Resistance | Excellent | Moderate |
| Cost | High | Low |
Introduction to Tungsten and Zinc Anodes
Tungsten anodes offer exceptional corrosion resistance and high melting points, making them ideal for robust industrial electrochemical applications. Zinc anodes provide cost-effective sacrificial protection with superior conductivity and are commonly used in marine and cathodic protection systems. Both metals serve critical roles in anode technology, chosen based on environmental conditions and specific electrochemical requirements.
Chemical and Physical Properties Comparison
Tungsten exhibits high melting point (3422degC) and exceptional corrosion resistance, making it highly stable as an anode material, while zinc has a lower melting point (419.5degC) and is more reactive, favoring sacrificial anode applications. Chemically, tungsten is inert in most environments, resisting oxidation and degradation, whereas zinc readily oxidizes, protecting metal structures through galvanic action. Physically, tungsten's high density (19.25 g/cm3) and hardness contrast with zinc's lower density (7.14 g/cm3) and malleability, influencing electrode lifespan and conductivity in electrochemical cells.
Electrochemical Performance: Tungsten vs Zinc
Tungsten anodes exhibit superior electrochemical performance with higher corrosion resistance and longer service life compared to zinc anodes, making them ideal for harsh environments. Zinc anodes provide lower overpotential and better initial current efficiency but corrode faster under aggressive conditions. The choice between tungsten and zinc anodes depends on specific application requirements such as durability, cost-effectiveness, and electrochemical stability.
Corrosion Resistance and Durability
Tungsten anodes offer superior corrosion resistance compared to zinc anodes, making them highly durable in harsh, acidic, or high-temperature environments. Zinc anodes tend to corrode more rapidly, limiting their lifespan and effectiveness in prolonged applications. The high melting point and density of tungsten contribute to its enhanced durability and stable electrochemical performance in corrosion protection systems.
Environmental Impact and Safety
Tungsten anodes exhibit a lower environmental impact due to their high recyclability and minimal leaching of toxic substances compared to zinc anodes, which can release harmful zinc ions into aquatic ecosystems. Zinc anodes pose safety concerns as their corrosion products may contribute to metal toxicity and water contamination, whereas tungsten's corrosion byproducts are generally less hazardous. Selecting tungsten anodes supports both environmental sustainability and enhanced safety in electrochemical applications.
Cost and Availability of Tungsten and Zinc
Zinc anodes are widely preferred in cathodic protection due to their low cost and high availability, making them economically feasible for large-scale use. Tungsten, although offering superior corrosion resistance and durability, is significantly more expensive and less abundant, resulting in higher material costs and limited accessibility. The cost-effectiveness of zinc combined with its ready availability largely outweighs the premium pricing and scarcity of tungsten in anode applications.
Application Suitability: Industry Use Cases
Tungsten anodes excel in high-temperature environments and aerospace applications due to their exceptional melting point and corrosion resistance. Zinc anodes are widely used in marine and construction industries for sacrificial protection against metal corrosion in seawater and soil. The choice between tungsten and zinc anodes depends on operational temperature, corrosion conditions, and industry-specific durability requirements.
Performance in Different Electrolytes
Tungsten anodes exhibit superior corrosion resistance and durability in acidic electrolytes compared to zinc anodes, which tend to degrade faster under such conditions. Zinc anodes perform effectively in alkaline electrolytes due to their higher electrochemical reactivity, offering efficient current output and lower polarization. Performance variability in different electrolytes is driven by tungsten's stable oxide layer formation, whereas zinc's reactivity leads to faster consumption but better initial conductivity in alkaline media.
Lifespan and Maintenance Considerations
Tungsten anodes exhibit a longer lifespan than zinc anodes due to their higher corrosion resistance and durability in harsh marine environments. Zinc anodes require more frequent replacement and maintenance because they erode faster, particularly in saltwater conditions. Maintenance considerations favor tungsten for cost-efficiency and reduced downtime, making it ideal for applications demanding extended protection and minimal upkeep.
Conclusion: Choosing the Right Anode Material
Tungsten anodes offer superior corrosion resistance and longer lifespan compared to zinc, making them ideal for high-performance and marine applications. Zinc anodes provide cost-effective protection and excellent galvanic properties, suited for general-purpose sacrificial anodes in freshwater environments. Selecting the right anode depends on environmental conditions, budget, and required durability for optimal corrosion protection.
Infographic: Tungsten vs Zinc for Anode
 azmater.com
azmater.com