Steel offers superior strength and durability, while zinc provides excellent corrosion resistance as a coating for galvanization. Zinc's sacrificial properties protect steel by preventing rust and extending the lifespan of galvanized products.
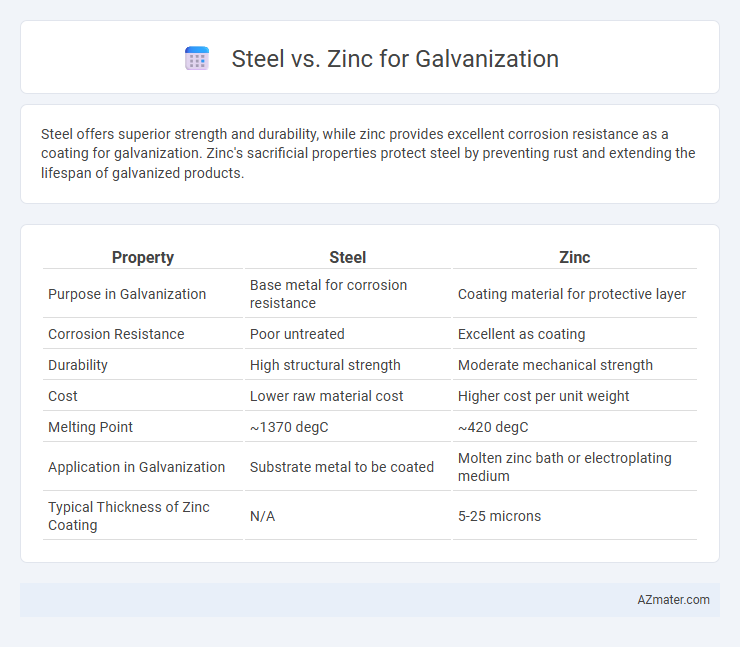
Table of Comparison
| Property | Steel | Zinc |
|---|---|---|
| Purpose in Galvanization | Base metal for corrosion resistance | Coating material for protective layer |
| Corrosion Resistance | Poor untreated | Excellent as coating |
| Durability | High structural strength | Moderate mechanical strength |
| Cost | Lower raw material cost | Higher cost per unit weight |
| Melting Point | ~1370 degC | ~420 degC |
| Application in Galvanization | Substrate metal to be coated | Molten zinc bath or electroplating medium |
| Typical Thickness of Zinc Coating | N/A | 5-25 microns |
Introduction to Galvanization
Galvanization is a corrosion protection process that involves applying a protective zinc coating to steel or iron to prevent rusting. Steel is the primary substrate for galvanization due to its susceptibility to corrosion, while zinc serves as the sacrificial metal offering galvanic protection. The key advantage of zinc coating on steel lies in its ability to protect both by barrier and cathodic protection, significantly extending the lifespan of steel structures in harsh environments.
Understanding Steel and Zinc Materials
Steel, an alloy primarily made of iron and carbon, offers high tensile strength and durability, making it a popular choice for construction and manufacturing. Zinc, a corrosion-resistant metal, is used in galvanization to form a protective layer that prevents rust on steel surfaces. Understanding the electrochemical properties of zinc and steel helps optimize the galvanization process for enhanced longevity in various environmental conditions.
The Science Behind Galvanization
Galvanization involves coating steel with zinc to protect against corrosion through a process called sacrificial protection, where zinc corrodes preferentially to steel, preserving the underlying metal. Zinc forms a tightly adhering layer of zinc oxide and zinc carbonate when exposed to air, creating a durable barrier that prevents moisture and oxygen from reaching the steel surface. The metallurgical bond between steel and zinc generated during hot-dip galvanization enhances mechanical adhesion and prolongs the lifespan of steel structures exposed to harsh environmental conditions.
Key Properties of Steel in Galvanization
Steel's high tensile strength and durability make it an ideal substrate for galvanization, providing a robust base that extends the protective coating's lifespan. Its ability to form a metallurgical bond with zinc enhances corrosion resistance, preventing rust and degradation in harsh environments. The steel's surface morphology and chemical composition significantly influence the adhesion and uniformity of the zinc layer, ensuring long-lasting protection.
Advantages of Zinc Coatings
Zinc coatings offer superior corrosion resistance by forming a protective barrier and sacrificial anode effect, significantly extending the lifespan of galvanized steel. The zinc layer also provides excellent adhesion for paint and other coatings, enhancing durability in harsh environments. Furthermore, zinc's self-healing properties allow minor scratches to repair themselves, preventing rust from spreading on steel surfaces.
Steel vs Zinc: Corrosion Resistance Comparisons
Steel coated with zinc exhibits superior corrosion resistance due to zinc's sacrificial anode properties, which protect the steel by corroding first. Zinc galvanization forms a stable, adherent layer that prevents rust formation on steel surfaces, significantly extending the lifespan of steel structures in harsh environments. Compared to uncoated steel, zinc-coated steel maintains structural integrity much longer, especially in outdoor and marine applications where exposure to moisture and salt accelerates corrosion.
Cost Factors: Steel and Zinc for Galvanization
Steel offers a cost-effective base material for galvanization, with prices generally lower than many alternative metals, making it a preferred choice for structural applications. Zinc, while more expensive than steel itself, provides a durable protective coating that extends the lifespan of steel by preventing corrosion, reducing long-term maintenance costs. The overall cost advantage of zinc-galvanized steel lies in combining steel's affordability with zinc's corrosion resistance, optimizing investment in both initial and future expenses.
Environmental Impact and Sustainability
Steel galvanization with zinc offers a durable protective layer that significantly reduces corrosion, extending the lifespan of steel products and minimizing resource consumption. Zinc coating is highly recyclable and requires less energy during production compared to alternative metals, contributing to lower greenhouse gas emissions. The environmental impact of zinc galvanization is mitigated by its long-lasting protection, reducing the frequency of steel replacement and waste generation in construction and industrial applications.
Common Applications in Industry
Steel is widely galvanized with zinc to enhance corrosion resistance in construction, automotive manufacturing, and infrastructure projects, where durability against rust is crucial. Zinc coatings provide a protective barrier for steel used in roofing, fencing, and outdoor equipment, extending the lifespan of these products in harsh environments. Industrial applications rely on galvanized steel for its cost-effectiveness and strength, making it ideal for pipelines, electrical transmission towers, and marine hardware.
Choosing the Right Material: Steel or Zinc?
Choosing the right material for galvanization involves understanding the distinct properties of steel and zinc, where steel provides structural strength while zinc offers superior corrosion resistance as a protective coating. Steel serves as the base metal, requiring galvanization with zinc to prevent rust and extend durability in various industrial applications. Prioritizing zinc coating thickness and steel grade ensures optimal performance for environments exposed to moisture and harsh conditions.
Infographic: Steel vs Zinc for Galvanization
 azmater.com
azmater.com