Shape memory alloys, such as Nitinol, offer superior flexibility and self-expanding properties for orthopedic implants compared to titanium alloys, which provide exceptional strength, corrosion resistance, and biocompatibility. The choice between these materials depends on the specific implant application, with shape memory alloys favored for dynamic load environments and titanium alloys preferred for load-bearing structural support.
Table of Comparison
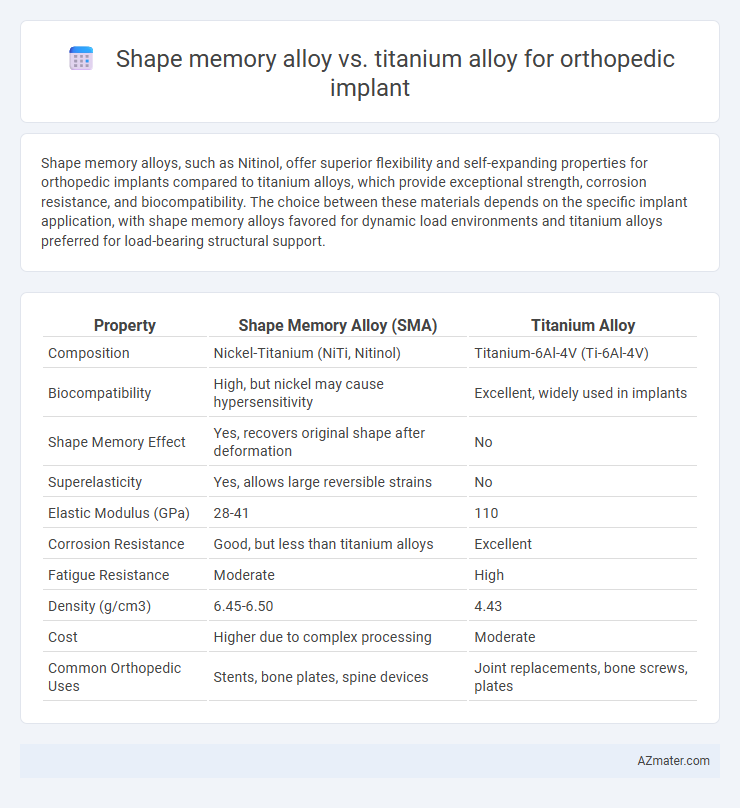
| Property | Shape Memory Alloy (SMA) | Titanium Alloy |
|---|---|---|
| Composition | Nickel-Titanium (NiTi, Nitinol) | Titanium-6Al-4V (Ti-6Al-4V) |
| Biocompatibility | High, but nickel may cause hypersensitivity | Excellent, widely used in implants |
| Shape Memory Effect | Yes, recovers original shape after deformation | No |
| Superelasticity | Yes, allows large reversible strains | No |
| Elastic Modulus (GPa) | 28-41 | 110 |
| Corrosion Resistance | Good, but less than titanium alloys | Excellent |
| Fatigue Resistance | Moderate | High |
| Density (g/cm3) | 6.45-6.50 | 4.43 |
| Cost | Higher due to complex processing | Moderate |
| Common Orthopedic Uses | Stents, bone plates, spine devices | Joint replacements, bone screws, plates |
Introduction to Orthopedic Implant Materials
Shape memory alloys (SMAs) and titanium alloys are pivotal materials in orthopedic implants due to their unique mechanical properties and biocompatibility. SMAs, such as Nitinol, exhibit superelasticity and shape memory effects, allowing for dynamic load accommodation and minimally invasive implantation. Titanium alloys, particularly Ti-6Al-4V, are favored for their high strength-to-weight ratio, corrosion resistance, and excellent osseointegration, making them ideal for permanent load-bearing implants.
Overview of Shape Memory Alloys
Shape memory alloys (SMAs), particularly nickel-titanium (Nitinol), are widely used in orthopedic implants due to their unique properties of superelasticity and shape memory effect, which enable them to return to their original shape after deformation, enhancing implant flexibility and durability. SMAs offer superior biocompatibility, corrosion resistance, and fatigue life compared to conventional metals, making them ideal for dynamic loading environments in orthopedic applications. Their ability to provide constant force and reduce stress shielding improves bone healing and implant integration compared to titanium alloys, which, while strong and lightweight, lack these distinct phase transformation properties.
Overview of Titanium Alloys
Titanium alloys are widely used in orthopedic implants due to their excellent biocompatibility, high strength-to-weight ratio, and corrosion resistance, which ensure long-term durability and patient safety. These alloys, primarily composed of titanium, aluminum, and vanadium (Ti-6Al-4V), offer superior osseointegration and mechanical properties that closely match human bone, reducing stress shielding and implant failure. Their widespread application in joint replacements, bone plates, and screws stems from their ability to maintain structural integrity under cyclic loading and harsh physiological environments.
Mechanical Properties Comparison
Shape memory alloys, such as Nitinol, exhibit superelasticity and excellent fatigue resistance, allowing implants to endure cyclic loading with minimal deformation, unlike titanium alloys which offer higher tensile strength and greater stiffness but less flexibility. Titanium alloys provide superior biocompatibility and corrosion resistance, ensuring long-term implant stability while shape memory alloys provide dynamic adaptability to physiological movements. The choice between these materials depends on the required balance between mechanical resilience, flexibility, and implant longevity in orthopedic applications.
Biocompatibility and Corrosion Resistance
Shape memory alloys, such as Nitinol, offer superior biocompatibility due to their unique ability to recover shape and reduce stress shielding, enhancing patient comfort and implant longevity. Titanium alloys are renowned for their exceptional corrosion resistance attributed to the stable titanium oxide layer, minimizing ion release and inflammatory response in the body. Both materials exhibit excellent biocompatibility, but titanium alloys generally provide better corrosion resistance, making them a preferred choice for long-term orthopedic implants.
Fatigue Performance and Longevity
Shape memory alloys, particularly Nitinol, exhibit superior fatigue resistance compared to titanium alloys, making them highly effective in load-bearing orthopedic implants where cyclic stresses are prevalent. Titanium alloys, while known for their high strength-to-weight ratio and excellent biocompatibility, may experience early fatigue failure under repetitive loading over long periods. The exceptional fatigue performance and ability to recover from deformation give shape memory alloys a notable advantage in enhancing implant longevity and maintaining structural integrity.
Elastic Modulus and Stress Shielding
Shape memory alloys (SMAs) such as Nitinol have an elastic modulus closer to that of natural bone (approximately 30-50 GPa) compared to titanium alloys (Ti-6Al-4V), which exhibit a higher elastic modulus of around 110 GPa. This lower elastic modulus in SMAs significantly reduces stress shielding, promoting better load transfer to the bone and minimizing bone resorption. Titanium alloys, though strong and biocompatible, can cause stress shielding due to their stiffness, potentially leading to implant loosening and compromised bone healing.
Clinical Applications and Case Studies
Shape memory alloys, particularly Nitinol, excel in orthodontic and small joint implant applications due to their superelasticity and biocompatibility, enabling dynamic movements and reducing implant failure rates documented in clinical studies. Titanium alloys, such as Ti-6Al-4V, dominate load-bearing orthopedic implants including hip and knee replacements, offering exceptional strength, corrosion resistance, and osseointegration verified by numerous case studies demonstrating long-term implant stability. Comparative clinical outcomes highlight shape memory alloys' advantage in minimally invasive procedures, while titanium alloys remain preferred for permanent fixation and durability in high-stress environments.
Cost-Effectiveness and Manufacturing
Shape memory alloys, such as NiTi (Nitinol), offer unique superelasticity and biocompatibility but are generally higher in cost due to complex manufacturing processes like precise alloying and heat treatment. Titanium alloys, widely used in orthopedic implants, provide excellent strength, corrosion resistance, and lower production costs through established casting and machining techniques. Cost-effectiveness favors titanium alloys for large-scale production, while shape memory alloys are preferred for specialized implants requiring adaptive mechanical performance.
Future Trends in Orthopedic Implant Materials
Shape memory alloys (SMAs) and titanium alloys are pivotal in advancing orthopedic implant technologies, with SMAs offering unique benefits such as superelasticity and thermal shape recovery, which enhance implant adaptability and patient comfort. Future trends focus on developing hybrid materials combining the biocompatibility and corrosion resistance of titanium alloys with the dynamic mechanical properties of SMAs to optimize load distribution and reduce implant failure rates. Innovations in nanoscale surface modifications and bioactive coatings are expected to improve osseointegration and long-term implant performance, driving the next generation of personalized and durable orthopedic solutions.
Infographic: Shape memory alloy vs Titanium alloy for Orthopedic implant
 azmater.com
azmater.com