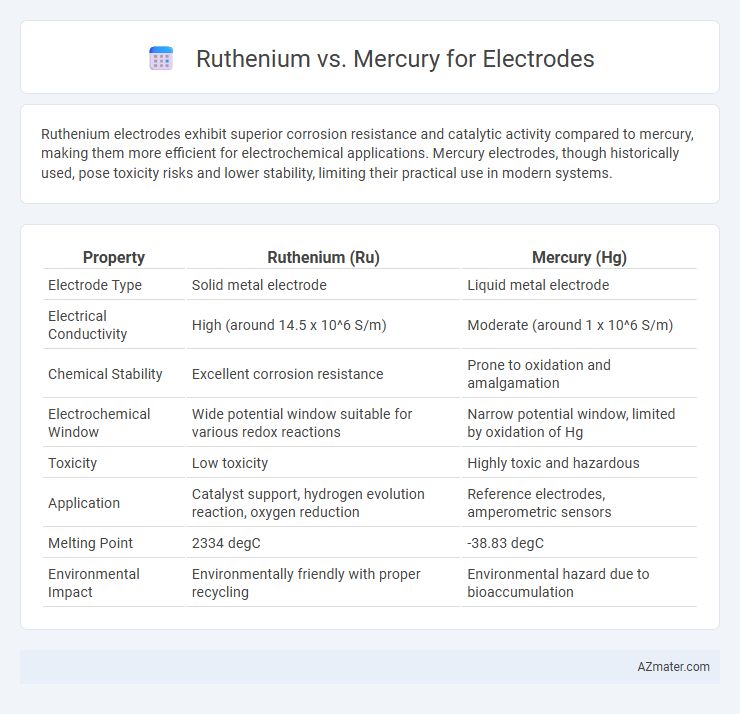
Ruthenium electrodes exhibit superior corrosion resistance and catalytic activity compared to mercury, making them more efficient for electrochemical applications. Mercury electrodes, though historically used, pose toxicity risks and lower stability, limiting their practical use in modern systems.
Table of Comparison
| Property | Ruthenium (Ru) | Mercury (Hg) |
|---|---|---|
| Electrode Type | Solid metal electrode | Liquid metal electrode |
| Electrical Conductivity | High (around 14.5 x 10^6 S/m) | Moderate (around 1 x 10^6 S/m) |
| Chemical Stability | Excellent corrosion resistance | Prone to oxidation and amalgamation |
| Electrochemical Window | Wide potential window suitable for various redox reactions | Narrow potential window, limited by oxidation of Hg |
| Toxicity | Low toxicity | Highly toxic and hazardous |
| Application | Catalyst support, hydrogen evolution reaction, oxygen reduction | Reference electrodes, amperometric sensors |
| Melting Point | 2334 degC | -38.83 degC |
| Environmental Impact | Environmentally friendly with proper recycling | Environmental hazard due to bioaccumulation |
Introduction to Ruthenium and Mercury Electrodes
Ruthenium electrodes exhibit exceptional corrosion resistance and catalytic activity, making them highly suitable for electrochemical applications such as water splitting and fuel cells. Mercury electrodes, historically used for their wide cathodic potential range and high overpotential for hydrogen evolution, offer unique advantages in electroanalytical chemistry despite toxicity concerns. The contrasting chemical properties and electrochemical behavior of ruthenium and mercury influence their selection for specific electrode applications in both industrial and research settings.
Chemical Properties: Ruthenium vs Mercury
Ruthenium exhibits excellent chemical stability and corrosion resistance, making it highly suitable for electrode materials in aggressive environments, while mercury is liquid at room temperature and prone to oxidation and amalgamation reactions. Ruthenium's high melting point (2334degC) and inertness to acids contrast with mercury's low melting point (-38.83degC) and susceptibility to forming amalgams with other metals. The superior catalytic properties of ruthenium enhance electrode performance in electrochemical reactions, whereas mercury's toxicity and volatility limit its use despite its historical application in electrodes.
Electrochemical Performance Comparison
Ruthenium exhibits superior electrochemical performance compared to mercury due to its higher catalytic activity, excellent stability, and better resistance to corrosion, making it ideal for electrodes in harsh environments. Mercury, while historically used for its wide cathodic potential range and good conductivity, suffers from toxicity and lower durability under prolonged electrochemical cycling. Ruthenium's enhanced electron transfer kinetics and robust surface chemistry result in improved current density and electrode lifespan, crucial for sustainable and efficient electrochemical applications.
Environmental Impact and Safety Concerns
Ruthenium electrodes exhibit lower environmental toxicity compared to mercury, as mercury is a highly toxic heavy metal with significant bioaccumulation risks and harmful effects on aquatic ecosystems. Ruthenium's chemical stability reduces the risk of hazardous leaching, making it a safer option in applications requiring electrode materials. Mercury electrodes pose serious safety concerns due to their volatility, vapor toxicity, and strict regulations limiting their use to prevent environmental contamination.
Cost-Effectiveness and Availability
Ruthenium electrodes offer superior durability and catalytic efficiency but come at a significantly higher cost and limited availability due to scarcity and complex extraction processes. Mercury electrodes are more affordable and readily available, with a lower initial investment, yet they pose environmental and health risks limiting their widespread use and regulatory acceptance. Cost-effectiveness favors ruthenium for long-term, high-performance applications, while mercury remains a budget-friendly choice for less demanding environments.
Applications in Analytical Chemistry
Ruthenium electrodes exhibit exceptional catalytic activity and chemical stability, making them ideal for electrochemical sensors and biosensors in analytical chemistry. Mercury electrodes, traditionally used in polarography and voltammetry, offer a wide cathodic potential range and reproducible surface but face toxicity and disposal limitations. Ruthenium's superior durability and environmentally friendly profile increasingly position it as a preferred material for trace metal analysis and electrochemical detection in modern analytical applications.
Durability and Longevity of Electrodes
Ruthenium electrodes exhibit superior durability and longevity compared to mercury electrodes due to their high corrosion resistance and stable electrochemical properties under harsh conditions. Ruthenium's ability to maintain catalytic activity over extended cycles reduces electrode degradation, ensuring consistent performance in electrolysis and fuel cell applications. Mercury electrodes, prone to oxidation and amalgamation, typically suffer from shorter operational lifespans and require more frequent replacement or maintenance.
Fabrication and Maintenance Processes
Ruthenium electrodes offer superior corrosion resistance and enhanced catalytic activity compared to mercury electrodes, resulting in longer operational lifespans and reduced maintenance requirements. The fabrication of ruthenium electrodes typically involves sputtering or electroplating techniques that provide uniform, adherent coatings, whereas mercury electrodes require careful handling due to mercury's toxicity and liquid state at room temperature. Maintenance processes for ruthenium electrodes are simplified by their stability and solid-state structure, contrasting with mercury electrodes that demand frequent rejuvenation and strict safety protocols to prevent contamination and exposure.
Regulatory Guidelines and Restrictions
Ruthenium electrodes are favored in regulatory frameworks due to their chemical stability and lower toxicity, aligning with strict environmental standards such as RoHS and REACH that limit hazardous substances in electronic components. Mercury electrodes face significant restrictions and bans under international regulations like the Minamata Convention because of mercury's high toxicity and environmental persistence, restricting their use in industrial and laboratory applications. Compliance with these guidelines drives the adoption of ruthenium over mercury in electrode manufacturing, ensuring safer and more sustainable practices.
Future Trends in Electrode Material Selection
Ruthenium is emerging as a preferred electrode material due to its superior corrosion resistance, higher catalytic activity, and longer lifespan compared to mercury, which is being phased out because of its toxicity and environmental hazards. Future trends in electrode material selection emphasize sustainable, non-toxic metals like ruthenium combined with nanostructured composites to enhance electrochemical performance and reduce environmental impact. Advanced manufacturing techniques such as atomic layer deposition are enabling precise ruthenium coatings that optimize conductivity and durability in energy storage and sensing applications.
Infographic: Ruthenium vs Mercury for Electrode
 azmater.com
azmater.com