Rhodium offers superior catalytic activity and selectivity in hydrogenation reactions compared to ruthenium, which excels in ammonia synthesis and oxidation processes due to its greater thermal stability. Rhodium's higher cost and scarcity are balanced by its efficiency, while ruthenium provides a cost-effective alternative with robust durability in harsh chemical environments.
Table of Comparison
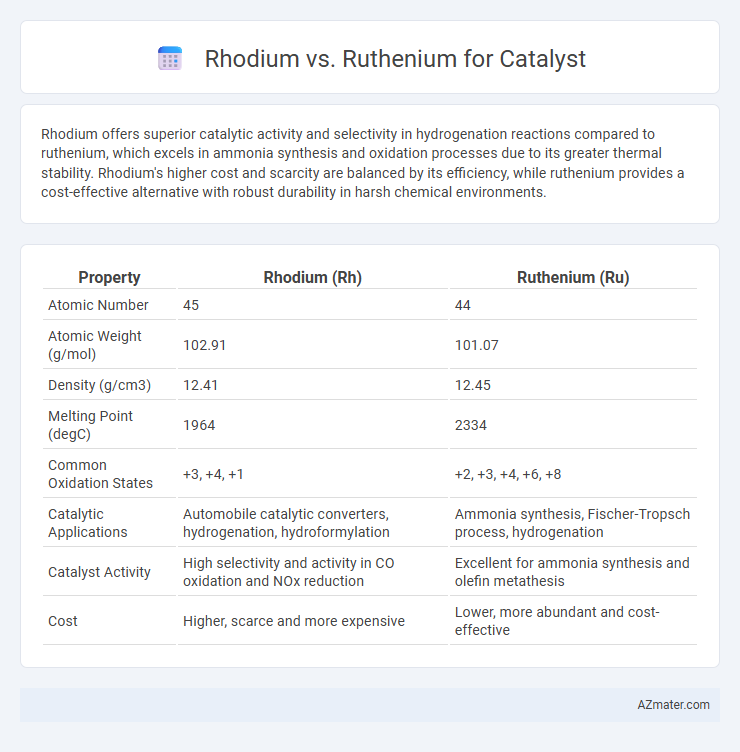
| Property | Rhodium (Rh) | Ruthenium (Ru) |
|---|---|---|
| Atomic Number | 45 | 44 |
| Atomic Weight (g/mol) | 102.91 | 101.07 |
| Density (g/cm3) | 12.41 | 12.45 |
| Melting Point (degC) | 1964 | 2334 |
| Common Oxidation States | +3, +4, +1 | +2, +3, +4, +6, +8 |
| Catalytic Applications | Automobile catalytic converters, hydrogenation, hydroformylation | Ammonia synthesis, Fischer-Tropsch process, hydrogenation |
| Catalyst Activity | High selectivity and activity in CO oxidation and NOx reduction | Excellent for ammonia synthesis and olefin metathesis |
| Cost | Higher, scarce and more expensive | Lower, more abundant and cost-effective |
Introduction to Rhodium and Ruthenium Catalysts
Rhodium and ruthenium are precious transition metals widely utilized as catalysts due to their exceptional activity and selectivity in chemical reactions. Rhodium catalysts excel in hydrogenation and hydroformylation processes, offering high efficiency in producing fine chemicals and pharmaceuticals. Ruthenium catalysts are favored for their versatility in oxidation reactions and olefin metathesis, contributing significantly to sustainable chemical synthesis and industrial applications.
Chemical Properties and Structure Comparison
Rhodium and ruthenium, both in the platinum group metals, exhibit distinct chemical properties influencing their catalytic performance. Rhodium displays superior resistance to oxidation and exceptional activity in hydrogenation reactions, attributed to its face-centered cubic (FCC) crystal structure which enhances electron density at active sites. Ruthenium's hexagonal close-packed (HCP) lattice allows for diverse oxidation states, making it highly effective in ammonia synthesis and oxidation catalysis due to its flexible electronic configuration and stronger metal-support interactions.
Catalytic Activity: Rhodium vs Ruthenium
Rhodium exhibits superior catalytic activity compared to ruthenium in hydrogenation and hydroformylation reactions due to its ability to activate molecular hydrogen more efficiently. Ruthenium, while also effective, shows enhanced performance in ammonia synthesis and Fischer-Tropsch processes because of its stronger nitrogen adsorption properties. The choice between rhodium and ruthenium catalysts depends on specific reaction conditions, substrate sensitivity, and desired product selectivity.
Selectivity in Chemical Reactions
Rhodium exhibits superior selectivity in hydrogenation and hydroformylation reactions, making it highly effective for producing specific isomers with minimal byproducts. Ruthenium catalysts, while versatile, typically show broader reactivity but lower selectivity, especially in ammonia synthesis and Fischer-Tropsch processes. Optimizing catalyst structure and support materials can enhance the selectivity of both metals in targeted chemical transformations.
Common Industrial Applications
Rhodium is widely used in automotive catalytic converters to reduce nitrogen oxides, carbon monoxide, and hydrocarbons emissions, playing a crucial role in pollution control. Ruthenium finds extensive application in chemical synthesis, particularly in olefin metathesis and hydrogenation reactions, due to its excellent catalytic activity and selectivity. Both metals contribute significantly to the production of fine chemicals, pharmaceuticals, and petrochemical processes, with rhodium favored for environmental catalysts and ruthenium for versatile organic transformations.
Cost and Availability of Rhodium and Ruthenium
Rhodium is significantly more expensive than ruthenium due to its rarity and limited global supply, with prices often reaching tens of thousands of dollars per ounce. Ruthenium is relatively more abundant, making it a cost-effective alternative for catalytic applications where budget constraints are critical. Availability of ruthenium is better supported by larger mining operations primarily in South Africa and Russia, whereas rhodium's supply is more volatile and dependent on the platinum group metals market.
Environmental Impact and Sustainability
Rhodium catalysts demonstrate high efficiency in automotive catalytic converters but involve energy-intensive extraction processes that contribute to environmental degradation and are less abundant, raising sustainability concerns. Ruthenium offers a more sustainable alternative due to its greater natural abundance and lower toxicity, presenting potential for eco-friendly catalytic applications with reduced environmental footprint. Advances in ruthenium-based catalysts promote greener chemical reactions and enhanced recyclability, positioning ruthenium as a promising candidate for sustainable catalyst development.
Stability and Longevity as Catalysts
Rhodium exhibits superior stability and longevity as a catalyst due to its high resistance to oxidation and thermal degradation, making it ideal for extended catalytic cycles in harsh environments. Ruthenium, while active, tends to form volatile oxides at elevated temperatures, which can reduce its catalytic lifespan and efficacy over time. Consequently, rhodium's enhanced durability under oxidative and high-temperature conditions often results in longer catalyst service life compared to ruthenium.
Recent Advances in Catalyst Technology
Recent advances in catalyst technology highlight rhodium and ruthenium's distinct roles in enhancing catalytic efficiency and selectivity. Rhodium catalysts exhibit exceptional performance in hydrogenation and carbon-carbon bond formation due to their unique electronic properties and stability under harsh conditions. Ruthenium catalysts demonstrate superior activity in ammonia synthesis and olefin metathesis, driven by innovations in nanoparticle design and support materials that optimize surface interactions.
Choosing the Right Metal: Application-Specific Recommendations
Rhodium excels in catalytic converters and hydrogenation reactions due to its superior resistance to oxidation and high activity at elevated temperatures, making it ideal for automotive and fine chemical industries. Ruthenium offers cost-effective performance in ammonia synthesis and olefin metathesis, favored for its robustness under harsh conditions and ability to catalyze carbon-carbon bond formation. Selecting the right metal requires assessing reaction environment, desired selectivity, and budget constraints to optimize catalyst efficiency and lifespan.
Infographic: Rhodium vs Ruthenium for Catalyst
 azmater.com
azmater.com