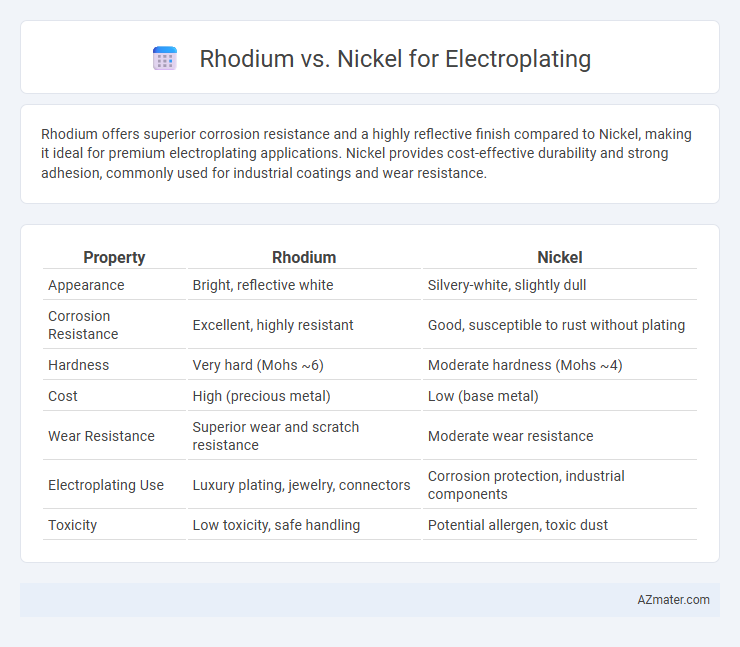
Rhodium offers superior corrosion resistance and a highly reflective finish compared to Nickel, making it ideal for premium electroplating applications. Nickel provides cost-effective durability and strong adhesion, commonly used for industrial coatings and wear resistance.
Table of Comparison
| Property | Rhodium | Nickel |
|---|---|---|
| Appearance | Bright, reflective white | Silvery-white, slightly dull |
| Corrosion Resistance | Excellent, highly resistant | Good, susceptible to rust without plating |
| Hardness | Very hard (Mohs ~6) | Moderate hardness (Mohs ~4) |
| Cost | High (precious metal) | Low (base metal) |
| Wear Resistance | Superior wear and scratch resistance | Moderate wear resistance |
| Electroplating Use | Luxury plating, jewelry, connectors | Corrosion protection, industrial components |
| Toxicity | Low toxicity, safe handling | Potential allergen, toxic dust |
Introduction to Electroplating
Electroplating involves depositing a thin metal coating on a substrate through an electrochemical process that enhances surface properties like corrosion resistance and aesthetic appeal. Rhodium and nickel are common plating materials; rhodium offers superior hardness, corrosion resistance, and a bright, reflective finish, while nickel provides excellent adhesion, durability, and cost-effectiveness. Selecting between rhodium and nickel depends on application requirements such as wear resistance, conductivity, and budget constraints in the electroplating process.
Overview of Rhodium and Nickel
Rhodium is a rare, silvery-white metal known for its exceptional hardness, high reflectivity, and superior corrosion resistance, making it ideal for electroplating applications that require a bright, durable finish. Nickel, a more abundant and cost-effective metal, offers excellent adhesion, corrosion resistance, and moderate hardness, commonly used to enhance the durability and functionality of plated surfaces. Both metals serve distinct electroplating purposes: rhodium for high-end decorative and protective coatings, and nickel for robust, corrosion-resistant layers in industrial applications.
Key Chemical Properties
Rhodium exhibits exceptional corrosion resistance and high reflectivity due to its noble metal status and atomic number 45, making it ideal for durable, bright electroplated finishes. Nickel, with atomic number 28, offers strong adhesion and hardness but is more prone to oxidation and corrosion compared to rhodium. The primary chemical difference lies in rhodium's inert nature and resistance to acids, whereas nickel forms protective oxide layers enhancing durability in less demanding environments.
Electroplating Process Differences
Rhodium electroplating requires a specialized plating bath with a low pH and uses a precious metal that provides exceptional corrosion and wear resistance. Nickel electroplating employs an alkaline or acidic nickel salt solution, offering high ductility, excellent adhesion, and cost-effectiveness. The plating process for rhodium demands precise current density control and shorter plating times due to its thin, reflective finish, while nickel plating allows for thicker, more durable layers suitable for industrial applications.
Durability and Wear Resistance
Rhodium offers superior durability and exceptional wear resistance compared to nickel, making it ideal for high-wear applications in jewelry and automotive parts. Its hardness and corrosion-resistant properties preserve the plated surface's integrity under harsh conditions, outperforming nickel's relatively softer and more prone-to-wear nature. Nickel plating provides decent durability but tends to tarnish and wear faster, requiring more frequent maintenance and reapplication.
Appearance and Finish Quality
Rhodium electroplating offers a brilliant, mirror-like finish with exceptional brightness and a highly reflective surface, making it ideal for jewelry and high-end decorative applications. Nickel plating provides a smooth, lustrous appearance with moderate reflectivity and can be polished to a high shine, but generally lacks the intense brilliance and whiteness of rhodium. Rhodium's superior tarnish resistance and hardness contribute to a longer-lasting, more durable finish compared to nickel's comparatively softer and more oxidation-prone surface.
Corrosion and Tarnish Resistance
Rhodium offers superior corrosion and tarnish resistance compared to nickel, making it ideal for electroplating high-end jewelry and electronics where durability and shine are critical. Nickel, while more cost-effective, is prone to oxidation and can develop a dull patina over time, especially in harsh environments. Rhodium's inert properties prevent it from reacting with air or moisture, ensuring long-lasting protection and maintaining a bright, reflective surface.
Cost Comparison
Rhodium plating commands a significantly higher cost than nickel due to rhodium's rarity and superior corrosion resistance, with rhodium prices often exceeding $10,000 per ounce compared to nickel's roughly $20 per pound. The initial material expense for rhodium electroplating is substantially greater, impacting overall production budgets, especially in high-volume applications. Nickel offers a cost-effective alternative with respectable durability, making it preferable for budget-conscious electroplating projects where extreme hardness and brightness of rhodium are less critical.
Common Applications
Rhodium is widely used in electroplating for automotive catalytic converters, jewelry, and electronic connectors due to its exceptional corrosion resistance and bright, reflective finish. Nickel electroplating is common in manufacturing hardware, automotive parts, and household appliances because of its excellent wear resistance and ability to provide a smooth, protective surface. Both metals serve critical roles in enhancing durability and aesthetics across industrial and consumer applications.
Choosing the Right Metal for Your Project
Rhodium offers superior corrosion resistance and a brilliant, reflective finish ideal for high-end jewelry and automotive trim, while nickel provides excellent durability and affordability, making it suitable for industrial applications and electronics. The choice between rhodium and nickel depends on factors like desired aesthetic, budget, and exposure to environmental elements. Selecting rhodium ensures enhanced tarnish resistance and premium appearance, whereas nickel delivers robust protection and cost-effectiveness for large-scale or functional plating projects.
Infographic: Rhodium vs Nickel for Electroplating
 azmater.com
azmater.com