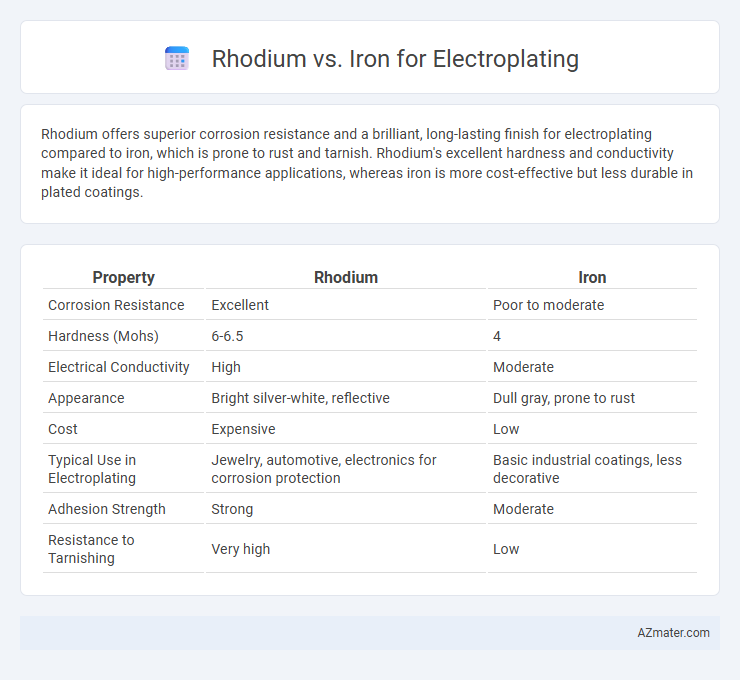
Rhodium offers superior corrosion resistance and a brilliant, long-lasting finish for electroplating compared to iron, which is prone to rust and tarnish. Rhodium's excellent hardness and conductivity make it ideal for high-performance applications, whereas iron is more cost-effective but less durable in plated coatings.
Table of Comparison
| Property | Rhodium | Iron |
|---|---|---|
| Corrosion Resistance | Excellent | Poor to moderate |
| Hardness (Mohs) | 6-6.5 | 4 |
| Electrical Conductivity | High | Moderate |
| Appearance | Bright silver-white, reflective | Dull gray, prone to rust |
| Cost | Expensive | Low |
| Typical Use in Electroplating | Jewelry, automotive, electronics for corrosion protection | Basic industrial coatings, less decorative |
| Adhesion Strength | Strong | Moderate |
| Resistance to Tarnishing | Very high | Low |
Introduction to Electroplating
Electroplating involves depositing a thin layer of metal, such as rhodium or iron, onto a substrate to enhance surface properties like corrosion resistance, conductivity, and aesthetics. Rhodium offers superior corrosion resistance, high reflectivity, and hardness, making it ideal for decorative and protective coatings in jewelry and electronics. Iron, while more economical and abundant, provides good adhesion and durability but lacks the corrosion resistance and luster of rhodium in electroplating applications.
Overview of Rhodium and Iron
Rhodium is a rare, silver-white metal known for its exceptional corrosion resistance, high reflectivity, and hardness, making it ideal for decorative and protective electroplating, especially in jewelry and automotive parts. Iron, a more abundant and cost-effective metal, offers good strength and magnetic properties but lacks the corrosion resistance and brightness of rhodium, often requiring additional coatings to enhance durability and appearance. Electroplating with rhodium produces a highly durable, tarnish-resistant finish, while iron plating is commonly used for cost-sensitive industrial applications where corrosion protection is moderate.
Electrochemical Properties Comparison
Rhodium exhibits superior corrosion resistance and higher catalytic activity compared to iron, making it ideal for durable, high-performance electroplating applications. Its higher standard reduction potential (+0.76 V for Rh3+/Rh) compared to iron's (-0.44 V for Fe2+/Fe) results in a more stable and vigorous deposition process. Iron's tendency to oxidize rapidly limits its plating quality and longevity, whereas rhodium's exceptional hardness and inertness ensure enhanced wear resistance and conductivity in electroplated coatings.
Aesthetic Results: Shine and Finish
Rhodium electroplating delivers a brilliant, mirror-like shine with exceptional reflectivity, providing a highly durable, tarnish-resistant finish ideal for luxury jewelry and high-end decorative pieces. Iron plating, while offering a sturdy and cost-effective protective layer, typically results in a duller, less reflective surface that lacks the luster and smooth finish associated with precious metal coatings. The superior brightness and corrosion resistance of rhodium plating make it the preferred choice when an exquisite aesthetic appearance is paramount.
Corrosion Resistance: Rhodium vs Iron
Rhodium offers superior corrosion resistance compared to iron due to its inertness and resistance to oxidation, making it highly effective in protecting surfaces from tarnishing and rust. Iron, while commonly used in electroplating, is prone to corrosion and requires protective coatings to prevent degradation. The exceptional corrosion resistance of rhodium makes it ideal for high-performance applications requiring durable and long-lasting finishes.
Durability and Wear Performance
Rhodium electroplating offers superior durability and exceptional wear resistance compared to iron, making it highly suitable for applications requiring long-lasting corrosion protection and scratch resistance. The dense, hard surface layer of rhodium significantly outperforms iron coatings, which tend to corrode and wear more quickly under mechanical stress. Rhodium's excellent chemical inertness and high hardness contribute to its enhanced lifespan in harsh environments, ensuring better performance for jewelry, automotive parts, and electronic components.
Cost Analysis of Rhodium and Iron
Rhodium electroplating is significantly more expensive than iron plating due to rhodium's rarity and high market price, often ranging between $6,000 to $10,000 per ounce compared to iron's minimal cost as a common industrial metal. The cost of rhodium plating also includes specialized equipment and skilled labor to handle its plating process, increasing overall expenses by up to 70% compared to iron. Iron electroplating remains the economical choice for large-scale applications with budget constraints, while rhodium is preferred for premium, corrosion-resistant finishes despite its high cost.
Common Industrial Applications
Rhodium is predominantly used in high-end electroplating applications such as jewelry, automotive catalytic converters, and electronic components due to its superior corrosion resistance, hardness, and reflective properties, providing an exceptionally durable and attractive finish. Iron electroplating is commonly employed in industrial applications requiring enhanced surface hardness and wear resistance, such as in heavy machinery, automotive parts, and tools, offering cost-effective protection but with less corrosion resistance compared to rhodium. The choice between rhodium and iron electroplating depends heavily on the specific industrial requirement for durability, visual appeal, and environmental resistance.
Environmental and Safety Considerations
Rhodium electroplating offers superior corrosion resistance and a non-toxic surface compared to iron, which can release hazardous iron oxides during plating. The use of rhodium reduces environmental impact due to its inertness and recyclability, whereas iron plating often involves toxic cyanide baths and produces more harmful waste byproducts. Handling and disposal protocols are less stringent for rhodium electroplating, enhancing workplace safety and minimizing ecological risks associated with iron plating processes.
Selecting the Best Metal for Electroplating
Rhodium outperforms iron in electroplating due to its superior corrosion resistance, high reflectivity, and exceptional hardness, ensuring long-lasting, bright finishes ideal for jewelry and electronics. Iron, while cost-effective and readily available, lacks the durability and luster required for premium coatings and is prone to rust and wear over time. Selecting rhodium for electroplating guarantees enhanced protection and aesthetic appeal compared to iron's limited functional and decorative benefits.
Infographic: Rhodium vs Iron for Electroplating
 azmater.com
azmater.com