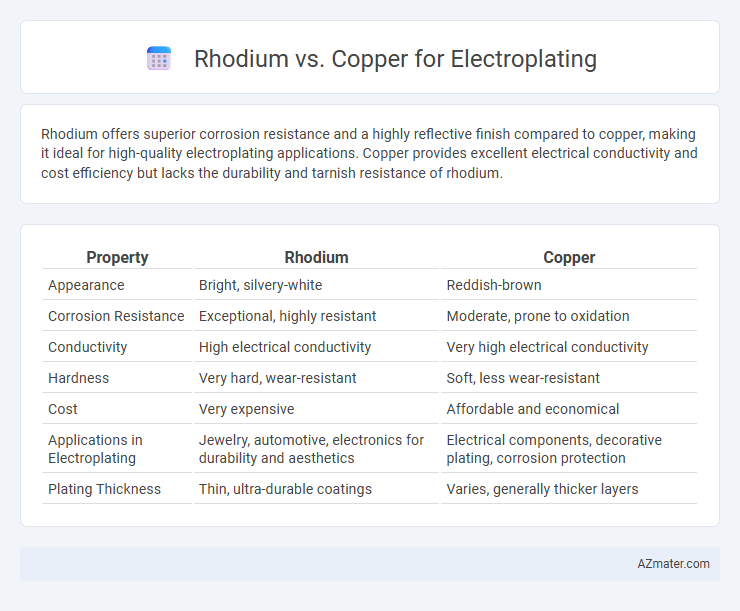
Rhodium offers superior corrosion resistance and a highly reflective finish compared to copper, making it ideal for high-quality electroplating applications. Copper provides excellent electrical conductivity and cost efficiency but lacks the durability and tarnish resistance of rhodium.
Table of Comparison
| Property | Rhodium | Copper |
|---|---|---|
| Appearance | Bright, silvery-white | Reddish-brown |
| Corrosion Resistance | Exceptional, highly resistant | Moderate, prone to oxidation |
| Conductivity | High electrical conductivity | Very high electrical conductivity |
| Hardness | Very hard, wear-resistant | Soft, less wear-resistant |
| Cost | Very expensive | Affordable and economical |
| Applications in Electroplating | Jewelry, automotive, electronics for durability and aesthetics | Electrical components, decorative plating, corrosion protection |
| Plating Thickness | Thin, ultra-durable coatings | Varies, generally thicker layers |
Introduction to Electroplating: Rhodium vs Copper
Electroplating involves depositing a metal layer onto a substrate to enhance corrosion resistance, conductivity, or aesthetics. Rhodium offers exceptional hardness, brilliant reflectivity, and superior corrosion resistance, making it ideal for jewelry and automotive parts. Copper excels in electrical conductivity and cost-effectiveness, commonly used for electronic components and PCB manufacturing.
Chemical Properties of Rhodium and Copper
Rhodium exhibits exceptional chemical inertness and resistance to corrosion, making it ideal for electroplating applications requiring durability and enhanced reflectivity. Copper, known for its excellent electrical conductivity and moderate corrosion resistance, offers a cost-effective plating solution but is prone to oxidation over time. The superior chemical stability of rhodium contributes to longer-lasting finishes compared to the more reactive surface of copper.
Electroplating Process Differences
Rhodium electroplating involves a more complex process using a rhodium sulfate or rhodium carbonate plating solution with precise current density control to achieve a thin, reflective, and corrosion-resistant coating. Copper electroplating typically uses copper sulfate and sulfuric acid bath solutions, allowing for thicker deposits with excellent conductivity and ductility. Rhodium plating requires shorter plating times due to the metal's high value and rarity, while copper plating supports longer deposition periods for enhancing electrical and mechanical properties.
Durability and Corrosion Resistance Comparison
Rhodium electroplating offers superior durability with a hardness rating of approximately 6 on the Mohs scale, significantly higher than copper's softer profile, making it more resistant to scratches and wear. In terms of corrosion resistance, rhodium's inert properties provide excellent protection against oxidation and tarnish, whereas copper is prone to developing a greenish patina due to oxidation over time. This makes rhodium plating ideal for applications requiring long-lasting, corrosion-resistant surfaces, especially in jewelry and automotive parts.
Cost Analysis: Rhodium vs Copper Plating
Rhodium plating commands significantly higher costs compared to copper plating due to its rarity and superior corrosion resistance, often priced at several hundred dollars per ounce versus copper's modest cost of less than a dollar per pound. Copper plating offers an economically efficient option for large-scale applications requiring moderate conductivity and corrosion protection, making it preferred in industries with tight budget constraints. The choice between rhodium and copper plating hinges on balancing budget limitations against performance demands, with rhodium favored for high-end jewelry and electronics where durability and appearance justify the premium expense.
Applications in Industry and Jewelry
Rhodium electroplating is extensively used in the jewelry industry to provide a highly reflective, corrosion-resistant finish that enhances the appearance and durability of silver and white gold pieces. Copper electroplating serves industrial applications by offering excellent electrical conductivity and corrosion resistance, making it ideal for electronic components and printed circuit boards. The superior hardness and tarnish resistance of rhodium compared to copper make it the preferred choice for luxury items, while copper's affordability and functional properties suit mass-production and industrial settings.
Aesthetic Results and Surface Finish
Rhodium electroplating offers a highly reflective, brilliant white finish that enhances the aesthetic appeal of jewelry and fine decorative pieces with superior tarnish resistance compared to copper. Copper plating provides a warm, reddish tone but is prone to oxidation and scratches, resulting in a less durable surface finish over time. The smoothness and brightness of rhodium plating create a premium, polished look favored in luxury applications, whereas copper's finish tends to dull and requires more maintenance to preserve its visual quality.
Electrical Conductivity Considerations
Rhodium offers excellent corrosion resistance and a bright finish but has lower electrical conductivity compared to copper, which limits its use in applications requiring efficient electrical conduction. Copper is widely favored in electroplating for electrical components due to its superior conductivity, nearly 97% of the International Annealed Copper Standard (IACS). When optimizing electroplated surfaces for electrical performance, copper's high conductivity ensures minimal energy loss, whereas rhodium is preferred for decorative or protective coatings where conductivity is less critical.
Environmental and Safety Impacts
Rhodium electroplating produces fewer hazardous byproducts compared to copper, making it a safer choice for minimizing environmental pollution and toxic waste. Copper electroplating involves the use of acidic solutions and can release harmful heavy metals and cyanides into wastewater, posing significant risks to aquatic life and human health. Proper waste treatment and containment are critical for copper plating facilities to mitigate environmental contamination, whereas rhodium plating typically demands less intensive hazardous material management.
Choosing the Right Metal for Your Electroplating Needs
Rhodium offers superior corrosion resistance, high reflectivity, and exceptional hardness, making it ideal for jewelry and high-wear applications, while copper provides excellent conductivity and a cost-effective base layer in multilayer electroplating systems. The choice depends on the desired finish quality, durability, and budget constraints, with rhodium favored for premium protection and aesthetics, and copper preferred for its affordability and conductive properties. Evaluating the specific requirements of your project ensures optimal metal selection for electroplating performance and longevity.
Infographic: Rhodium vs Copper for Electroplating
 azmater.com
azmater.com