Osmium offers exceptional electrical conductivity and durability, making it ideal for high-performance electronic contacts, while gallium provides unique semiconductor properties, essential for advanced integrated circuits and optoelectronic devices. The choice between osmium and gallium depends on whether durability or semiconductor functionality is prioritized in electronic applications.
Table of Comparison
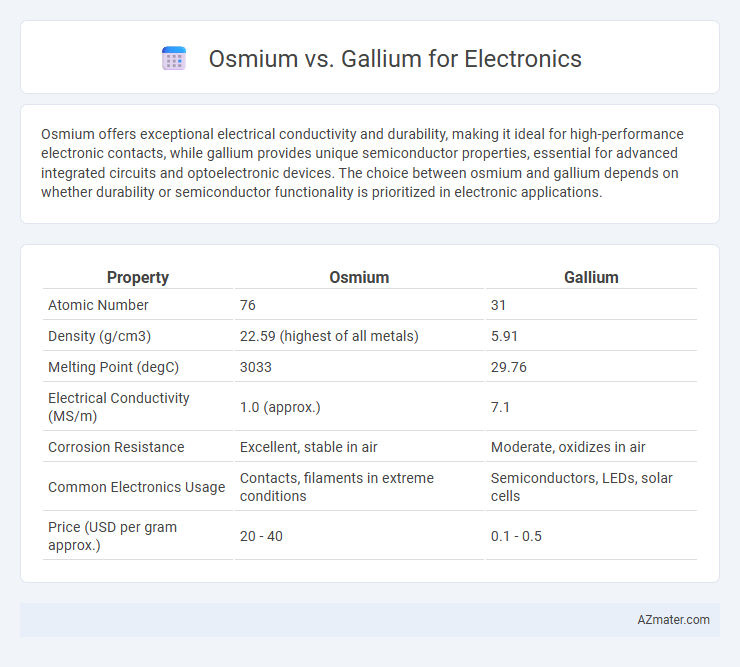
| Property | Osmium | Gallium |
|---|---|---|
| Atomic Number | 76 | 31 |
| Density (g/cm3) | 22.59 (highest of all metals) | 5.91 |
| Melting Point (degC) | 3033 | 29.76 |
| Electrical Conductivity (MS/m) | 1.0 (approx.) | 7.1 |
| Corrosion Resistance | Excellent, stable in air | Moderate, oxidizes in air |
| Common Electronics Usage | Contacts, filaments in extreme conditions | Semiconductors, LEDs, solar cells |
| Price (USD per gram approx.) | 20 - 40 | 0.1 - 0.5 |
Introduction to Osmium and Gallium in Electronics
Osmium and gallium serve distinct roles in electronics due to their unique physical and chemical properties. Osmium, known for its extreme density and durability, is primarily used in specialized electrical contacts and filaments where hardness and wear resistance are critical. Gallium, prized for its low melting point and semiconductor capabilities, is essential in the production of gallium arsenide (GaAs) and gallium nitride (GaN) components, widely applied in high-speed and optoelectronic devices.
Elemental Properties Comparison
Osmium exhibits the highest density (22.59 g/cm3) and exceptional hardness, making it ideal for durable electronic contacts and components requiring wear resistance. Gallium, with a low melting point of about 29.76degC and excellent semiconductor properties, is widely used in high-speed integrated circuits and optoelectronic devices. The stark contrast in melting points and electrical conductivity between osmium and gallium defines their specialized applications in electronics, from robust hardware elements to advanced semiconductor technology.
Electrical Conductivity Differences
Osmium exhibits significantly higher electrical conductivity than gallium, making it more suitable for applications requiring efficient electron flow. Gallium's lower conductivity is compensated by its unique semiconductor properties and flexibility in liquid state near room temperature. When choosing materials for electronics, osmium's superior conductivity supports high-performance circuits, whereas gallium excels in specialized roles like semiconductors and thermometers.
Melting Points and Thermal Stability
Osmium boasts a melting point of approximately 3,033degC, significantly higher than gallium's melting point of 29.76degC, making osmium far more suitable for electronics requiring extreme thermal stability. Gallium's low melting point allows it to transition from solid to liquid near room temperature, which limits its use in high-temperature electronic applications but enables unique applications like thermometers and semiconductors. Osmium's robustness under high heat enhances device longevity and performance in harsh environments, whereas gallium is ideal for low-power, flexible electronics due to its thermal conductivity and phase-change properties.
Corrosion Resistance and Longevity
Osmium exhibits exceptional corrosion resistance due to its dense, inert nature and high melting point, making it highly durable for electronics exposed to harsh environments. Gallium, though more reactive and prone to oxidation, offers unique conductivity advantages but requires protective coatings to ensure longevity in electronic components. The superior corrosion resistance of osmium significantly enhances the lifespan of devices in corrosive or high-temperature applications compared to gallium.
Semiconductor Applications and Performance
Osmium exhibits exceptional electrical conductivity and high melting point, making it suitable for high-temperature semiconductor applications requiring durability and stability. Gallium, particularly in the form of gallium arsenide (GaAs), offers superior electron mobility and direct bandgap properties essential for high-speed and optoelectronic devices. While osmium's robustness supports demanding environmental conditions, gallium-based semiconductors dominate in performance for integrated circuits and advanced electronic components.
Availability and Cost Analysis
Osmium, a rare and dense platinum-group metal, is scarce and extremely expensive, limiting its practical use in electronics despite its excellent conductivity and durability. Gallium, on the other hand, is more abundant, readily available as a byproduct of aluminum production, and significantly cheaper, making it a cost-effective choice for semiconductor applications such as LEDs and integrated circuits. The high cost and limited availability of osmium restrict its use to niche applications, whereas gallium's accessibility drives widespread adoption in the electronics industry.
Environmental and Safety Considerations
Osmium and gallium present distinct environmental and safety profiles in electronics, with osmium's extreme density and toxicity requiring careful handling to avoid harmful osmium tetroxide exposure. Gallium, used in semiconductors and LEDs, offers lower toxicity and less environmental risk, though its extraction can impact ecosystems due to mining activities. Selecting gallium typically aligns better with sustainable electronics manufacturing due to reduced hazard potential and safer disposal processes.
Current Industry Usage Trends
Osmium, known for its extreme density and high hardness, sees limited use in electronics primarily due to its rarity and cost, while gallium plays a critical role in modern semiconductor technology through its application in gallium arsenide (GaAs) and gallium nitride (GaN) components. Gallium-based materials dominate high-frequency and optoelectronic devices, enabling advancements in 5G networks, LED lighting, and power electronics, driving increased production and research investment. Current industry trends indicate gallium's growing importance for efficient, high-performance electronic components, whereas osmium remains niche, often restricted to specialized scientific equipment rather than mainstream electronics manufacturing.
Future Prospects in Electronic Technology
Osmium's exceptional electron density and durability make it a promising candidate for high-performance electrodes in advanced electronic devices, especially in nanoscale components requiring high conductivity and wear resistance. Gallium, with its low melting point and excellent semiconducting properties, is pivotal in developing flexible electronics and next-generation optoelectronic devices, particularly in gallium arsenide-based solar cells and LEDs. Future electronic technologies will likely leverage osmium's robustness for extreme environments and gallium's versatility for lightweight, flexible, and highly efficient semiconductor applications.
Infographic: Osmium vs Gallium for Electronics
 azmater.com
azmater.com