Rare earth metals offer superior magnetic and electrochemical properties for electronic components compared to silver, enabling enhanced performance in permanent magnets and capacitors. Silver provides excellent electrical conductivity but lacks the specialized functionalities essential in high-tech electronics where rare earth metals are crucial.
Table of Comparison
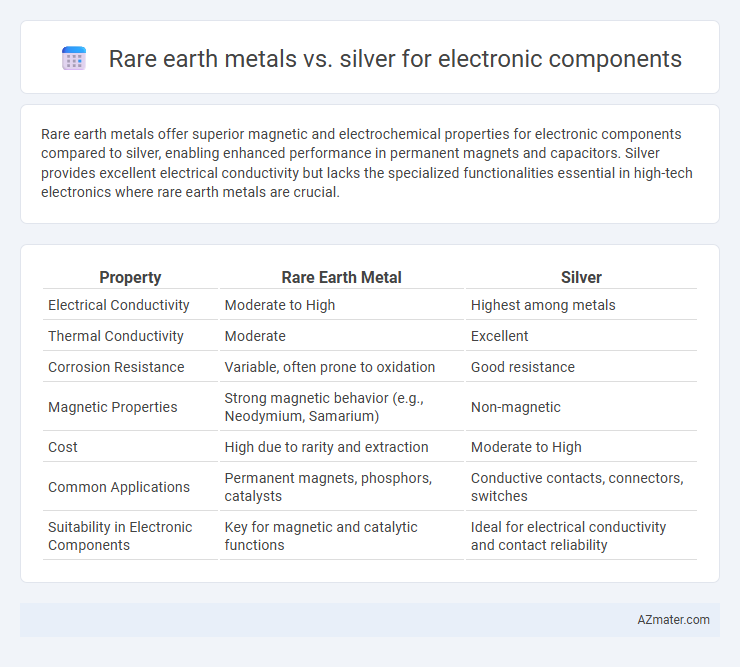
| Property | Rare Earth Metal | Silver |
|---|---|---|
| Electrical Conductivity | Moderate to High | Highest among metals |
| Thermal Conductivity | Moderate | Excellent |
| Corrosion Resistance | Variable, often prone to oxidation | Good resistance |
| Magnetic Properties | Strong magnetic behavior (e.g., Neodymium, Samarium) | Non-magnetic |
| Cost | High due to rarity and extraction | Moderate to High |
| Common Applications | Permanent magnets, phosphors, catalysts | Conductive contacts, connectors, switches |
| Suitability in Electronic Components | Key for magnetic and catalytic functions | Ideal for electrical conductivity and contact reliability |
Introduction to Rare Earth Metals and Silver in Electronics
Rare earth metals, including neodymium, europium, and terbium, are critical in electronic components for their exceptional magnetic, luminescent, and conductive properties, enabling high-performance magnets, phosphors, and batteries. Silver, known for its highest electrical conductivity among metals, is widely used in electronic contacts, conductors, and printed circuit boards to ensure efficient signal transmission and minimal energy loss. The choice between rare earth metals and silver depends on specific device requirements such as magnetic strength, conductivity, and cost-effectiveness in electronics manufacturing.
Composition and Properties of Rare Earth Metals
Rare earth metals, primarily composed of 17 elements including scandium, yttrium, and the lanthanides, exhibit unique magnetic, luminescent, and catalytic properties essential for advanced electronic components. In contrast, silver, a single element with exceptional electrical conductivity and thermal properties, lacks the rare earth metals' ability to enhance magnetism and optical functions crucial in modern electronics. The composition of rare earth metals imparts superior performance in components such as permanent magnets, phosphors, and battery electrodes, enabling innovations beyond silver's traditional conductive applications.
Silver: Characteristics and Electronic Benefits
Silver stands out in electronic components due to its highest electrical conductivity among metals, facilitating efficient current flow and superior signal integrity. Its excellent thermal conductivity helps in effective heat dissipation, reducing device overheating and enhancing longevity. Unlike some rare earth metals, silver offers exceptional corrosion resistance and solderability, making it ideal for high-performance connectors, contacts, and conductive inks in various electronic applications.
Conductivity Comparison: Rare Earth Metals vs. Silver
Silver exhibits superior electrical conductivity with a resistivity of approximately 1.59 x 10^-8 ohm meters, outperforming most rare earth metals which typically have higher resistivities and lower electron mobility. Rare earth metals like neodymium or dysprosium are primarily valued for magnetic and catalytic properties rather than conductivity, limiting their application in electronic components where efficient current flow is critical. Despite unique chemical and magnetic features, rare earth metals cannot match silver's exceptional conductivity, making silver the preferred choice for high-performance electrical contacts and interconnects.
Applications in Electronic Components
Rare earth metals, such as neodymium and dysprosium, are essential in electronic components for their superior magnetic and conductive properties, enabling high-performance permanent magnets in hard drives, speakers, and advanced sensors. Silver offers exceptional electrical conductivity and is widely used in conductive inks, printed circuit boards (PCBs), and high-frequency electronic applications to ensure efficient signal transmission. Both materials are critical in electronics, with rare earth metals enhancing magnetic and electronic performance, while silver provides optimal electrical conductivity and corrosion resistance.
Cost Analysis: Economic Impact of Material Choice
Rare earth metals generally exhibit higher cost volatility compared to silver, primarily due to their concentrated supply chains in regions like China. Silver offers more stable pricing and lower extraction costs, making it economically favorable for mass-produced electronic components. However, rare earth metals provide unique magnetic and conductive properties essential for high-performance applications despite their higher material expenses.
Reliability and Durability in Device Performance
Rare earth metals exhibit superior reliability and durability in electronic components due to their exceptional magnetic, thermal, and corrosion-resistant properties, enhancing device lifespan and stable performance under extreme conditions. Silver, while offering excellent electrical conductivity, tends to suffer from tarnishing and electromigration, which can reduce long-term component reliability and lead to performance degradation. The choice between rare earth metals and silver depends on the criticality of device operating environment and longevity requirements, with rare earth elements often preferred for high-reliability applications.
Environmental and Ethical Considerations
Rare earth metals, essential in many electronic components, pose significant environmental risks due to toxic mining processes and radioactive waste production, whereas silver extraction generally has a lower environmental impact but still involves energy-intensive mining and water use. Ethical concerns surrounding rare earth metals include child labor and poor working conditions in supply chains, contrasting with relatively more regulated silver mining industries. Sustainable sourcing and recycling initiatives are critical to mitigating the environmental footprint and addressing ethical challenges in both rare earth metal and silver use for electronics.
Future Trends in Electronic Material Selection
Rare earth metals exhibit superior magnetic, luminescent, and catalytic properties essential for advanced electronic components, driving their increasing adoption in next-generation devices over silver, which primarily excels in electrical conductivity. Emerging trends indicate a shift towards rare earth elements such as neodymium and dysprosium for high-performance magnets and phosphors in displays, enhancing miniaturization and energy efficiency. Sustainability concerns and supply chain challenges prompt research into recycling and alternative materials, positioning rare earth metals as crucial yet strategically managed resources in future electronic material selection.
Conclusion: Choosing Between Rare Earth Metals and Silver
Rare earth metals offer superior magnetic, luminescent, and catalytic properties essential for high-performance electronic components, particularly in advanced applications like permanent magnets and phosphors. Silver provides exceptional electrical conductivity and antimicrobial properties, making it ideal for circuit connections and contacts in consumer electronics. Selecting between rare earth metals and silver depends on specific device requirements, balancing conductivity and specialty functions against cost and availability.
Infographic: Rare earth metal vs Silver for Electronic component
 azmater.com
azmater.com