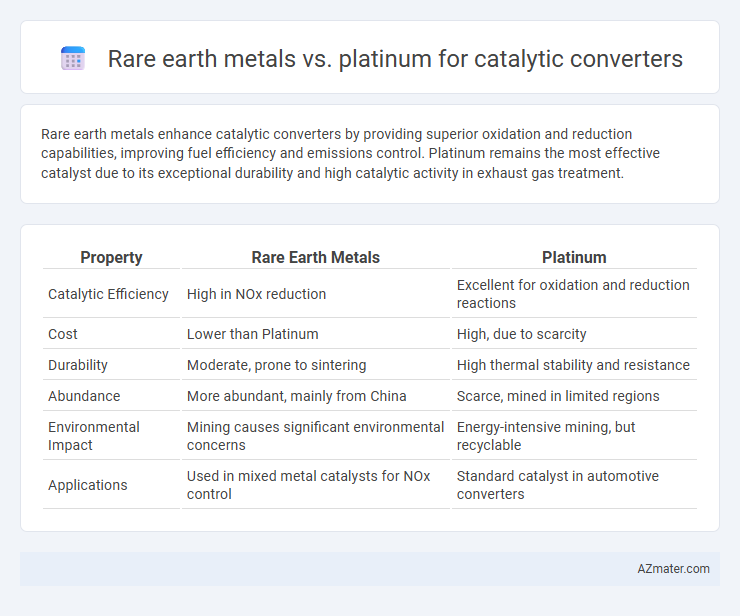
Rare earth metals enhance catalytic converters by providing superior oxidation and reduction capabilities, improving fuel efficiency and emissions control. Platinum remains the most effective catalyst due to its exceptional durability and high catalytic activity in exhaust gas treatment.
Table of Comparison
| Property | Rare Earth Metals | Platinum |
|---|---|---|
| Catalytic Efficiency | High in NOx reduction | Excellent for oxidation and reduction reactions |
| Cost | Lower than Platinum | High, due to scarcity |
| Durability | Moderate, prone to sintering | High thermal stability and resistance |
| Abundance | More abundant, mainly from China | Scarce, mined in limited regions |
| Environmental Impact | Mining causes significant environmental concerns | Energy-intensive mining, but recyclable |
| Applications | Used in mixed metal catalysts for NOx control | Standard catalyst in automotive converters |
Introduction to Catalytic Converters
Catalytic converters rely on rare earth metals such as cerium and lanthanum for oxygen storage and improving catalytic efficiency, while platinum serves as a highly effective catalyst in facilitating the oxidation and reduction of harmful vehicle emissions. Rare earth elements enhance durability and thermal stability, aiding in the continuous conversion of carbon monoxide, hydrocarbons, and nitrogen oxides into less toxic gases. Platinum's exceptional catalytic properties and resistance to poisoning make it indispensable in achieving stringent emission standards in automotive exhaust systems.
What Are Rare Earth Metals?
Rare earth metals, including elements like cerium, neodymium, and lanthanum, are critical components in catalytic converters due to their exceptional catalytic properties and abundance compared to platinum. These metals enhance the efficiency of catalytic converters by facilitating oxidation-reduction reactions that reduce harmful vehicle emissions. Unlike platinum, rare earth metals offer a cost-effective and abundant alternative while maintaining high catalytic performance essential for emission control in modern automotive technology.
Platinum: The Classic Catalyst
Platinum remains the classic catalyst in catalytic converters due to its exceptional ability to facilitate oxidation and reduction reactions, effectively reducing harmful emissions like carbon monoxide and nitrogen oxides. While rare earth metals can enhance catalyst performance by improving thermal stability and oxygen storage capacity, platinum's high catalytic activity and durability make it indispensable in automotive emissions control. The superior resistance of platinum to poisoning and sintering under harsh exhaust conditions solidifies its dominant role in catalytic converter technology.
Key Roles in Emission Control
Rare earth metals like cerium and lanthanum enhance catalytic converters by promoting oxygen storage and release, improving the oxidation of carbon monoxide and hydrocarbons. Platinum acts as a primary catalyst facilitating redox reactions critical for converting harmful nitrogen oxides into benign nitrogen and oxygen gases. Combining rare earth metals with platinum significantly boosts catalytic efficiency and durability in emission control systems.
Efficiency: Rare Earth Metals vs. Platinum
Rare earth metals such as cerium and lanthanum exhibit superior oxygen storage capacity, enhancing catalytic converter efficiency through improved oxidation and reduction reactions compared to platinum. Platinum remains highly effective in facilitating catalytic reactions but is more susceptible to poisoning and deactivation under certain exhaust conditions. Incorporating rare earth metals into catalytic converters increases durability and performance while potentially reducing reliance on costly platinum.
Cost Comparison and Market Trends
Rare earth metals, primarily cerium and lanthanum, are increasingly favored in catalytic converters due to their lower cost compared to platinum, which remains significantly more expensive per ounce. Market trends show a growing demand for rare earth elements driven by their abundant supply and effectiveness in reducing emissions, which helps manufacturers lower production costs while meeting stricter environmental regulations. Platinum prices fluctuate with global mining outputs and geopolitical factors, causing manufacturers to shift towards rare earth metals to stabilize costs and ensure sustainable sourcing.
Environmental Impact of Each Material
Rare earth metals used in catalytic converters, such as cerium and lanthanum, offer superior oxidation-reduction efficiency but pose environmental challenges due to mining toxicity and radioactive waste byproducts. Platinum, a noble metal, provides excellent catalytic performance with high durability and lower toxicity risk but requires intensive mining processes that contribute to habitat destruction and significant carbon emissions. Both materials necessitate recycling initiatives to mitigate environmental impact and conserve finite resources, with rare earth element extraction demanding stricter regulations to minimize ecological damage.
Availability and Supply Chain Concerns
Rare earth metals such as cerium and lanthanum are more abundant and geographically diverse compared to platinum, which is concentrated in limited regions like South Africa and Russia, making rare earths less susceptible to geopolitical supply disruptions. However, rare earth extraction involves complex environmental challenges and processing bottlenecks that can constrain supply chain stability. Platinum's scarcity and high demand in automotive catalytic converters have resulted in volatile prices and reliance on a brittle supply chain, prompting manufacturers to seek alternative materials with more reliable availability.
Advances in Catalyst Technology
Advances in catalyst technology have seen rare earth metals like cerium and lanthanum increasingly incorporated into catalytic converters to enhance oxygen storage capacity and improve pollutant oxidation efficiency. Platinum remains a crucial component due to its superior catalytic activity and durability, but combining it with rare earth elements boosts overall performance while reducing platinum loading and cost. These innovations result in more sustainable and cost-effective catalytic converters with improved emission control in modern vehicles.
Future Outlook: Sustainability and Innovations
Rare earth metals, essential for catalytic converters due to their superior oxygen storage capacity and cost-effectiveness, face increasing supply chain risks that drive research into sustainable recycling and alternative materials. Platinum, prized for its high catalytic efficiency and stability, remains scarce and expensive, prompting innovations in nano-engineering and reduced loading techniques to enhance performance while minimizing environmental impact. Future advancements are expected to blend rare earth metal recycling with platinum group metal optimization, fostering eco-friendly, durable catalytic converters aligned with stringent emission regulations.
Infographic: Rare earth metal vs Platinum for Catalytic Converter
 azmater.com
azmater.com