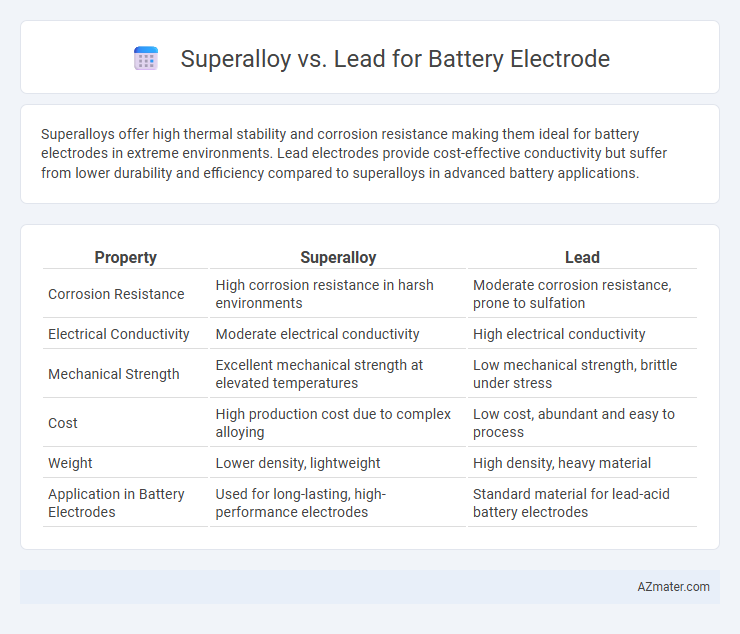
Superalloys offer high thermal stability and corrosion resistance making them ideal for battery electrodes in extreme environments. Lead electrodes provide cost-effective conductivity but suffer from lower durability and efficiency compared to superalloys in advanced battery applications.
Table of Comparison
| Property | Superalloy | Lead |
|---|---|---|
| Corrosion Resistance | High corrosion resistance in harsh environments | Moderate corrosion resistance, prone to sulfation |
| Electrical Conductivity | Moderate electrical conductivity | High electrical conductivity |
| Mechanical Strength | Excellent mechanical strength at elevated temperatures | Low mechanical strength, brittle under stress |
| Cost | High production cost due to complex alloying | Low cost, abundant and easy to process |
| Weight | Lower density, lightweight | High density, heavy material |
| Application in Battery Electrodes | Used for long-lasting, high-performance electrodes | Standard material for lead-acid battery electrodes |
Introduction to Battery Electrode Materials
Battery electrode materials significantly influence energy storage efficiency and lifespan, with superalloys and lead presenting distinct advantages. Superalloys offer exceptional corrosion resistance and mechanical strength, enhancing battery durability under high-stress conditions. Conversely, lead, widely used in lead-acid batteries, provides cost-effectiveness and reliable electrochemical properties essential for mainstream energy storage applications.
What Are Superalloys?
Superalloys are high-performance metal alloys primarily composed of nickel, cobalt, or iron, designed to withstand extreme temperatures and corrosive environments, making them ideal for advanced battery electrodes. Their superior thermal stability and mechanical strength enable enhanced battery efficiency and durability compared to lead-based electrodes. Unlike lead, superalloys offer improved conductivity and resistance to degradation, contributing to longer battery life and better performance in demanding applications.
Lead as a Traditional Electrode Material
Lead has been the traditional electrode material in battery technology due to its excellent corrosion resistance and reliable electrochemical performance in lead-acid batteries. Its high conductivity and ability to undergo reversible oxidation-reduction reactions make it ideal for energy storage applications, despite its weight and environmental concerns. Lead electrodes offer cost-effectiveness and a well-established manufacturing process, maintaining their relevance in many industrial battery systems compared to newer superalloy alternatives.
Key Performance Metrics: Superalloy vs Lead
Superalloys exhibit superior corrosion resistance and higher temperature stability compared to lead, making them ideal for advanced battery electrodes in high-demand applications. Lead electrodes provide cost efficiency and excellent conductivity but suffer from lower cycle life and susceptibility to sulfation. Key performance metrics highlight superalloys' enhanced durability and energy density, whereas lead excels in affordability and established recycling infrastructure.
Energy Density Comparison
Superalloy battery electrodes exhibit significantly higher energy density compared to lead-based electrodes due to their superior electrochemical stability and ability to operate at elevated temperatures. The enhanced energy density of superalloy electrodes enables longer battery life and improved power output, making them ideal for high-performance applications such as aerospace and electric vehicles. In contrast, lead electrodes typically offer lower energy density and are limited by their weight and slower electrochemical kinetics.
Durability and Lifespan Analysis
Superalloys exhibit superior durability and extended lifespan compared to lead when used in battery electrodes due to their high resistance to corrosion, mechanical stress, and thermal degradation. Lead electrodes tend to suffer from sulfation and corrosion, which significantly reduce their operational life and efficiency under cycling conditions. The enhanced mechanical strength and chemical stability of superalloy electrodes result in prolonged battery performance and reduced maintenance costs over time.
Environmental Impact and Safety
Superalloys, composed primarily of nickel, cobalt, and iron, offer superior corrosion resistance and stability compared to lead, resulting in lower environmental contamination risks during battery use and disposal. Lead electrodes pose significant environmental hazards due to lead's toxicity and potential for soil and water pollution, demanding stringent recycling and handling protocols to mitigate health risks. Advanced superalloy electrodes reduce hazardous waste generation and improve worker safety by minimizing exposure to toxic heavy metals in battery manufacturing and recycling processes.
Cost and Availability Factors
Superalloys, composed primarily of nickel, cobalt, and iron, offer superior corrosion resistance and high-temperature stability compared to lead but come at a significantly higher cost due to complex extraction and refining processes. Lead remains widely available and cost-effective, benefiting from abundant global reserves and established recycling infrastructure, making it a preferred choice for large-scale battery electrodes. While superalloys provide enhanced performance, their limited availability and elevated price restrict their adoption in cost-sensitive battery applications.
Applications in Modern Battery Technologies
Superalloy electrodes, composed of nickel, cobalt, and chromium, offer superior corrosion resistance and mechanical strength, making them ideal for high-performance lithium-ion and solid-state batteries used in electric vehicles and aerospace applications. Lead electrodes, primarily utilized in traditional lead-acid batteries, provide cost-effective energy storage with reliable cycling performance but suffer from lower energy density and limited lifespan, restricting their use to automotive starters and stationary backup power. Modern battery technologies increasingly favor superalloy materials for enhanced durability and energy efficiency, driving advancements in portable electronics and renewable energy storage systems.
Future Prospects: Superalloy or Lead?
Superalloys, with their superior corrosion resistance and mechanical strength, offer promising advantages for next-generation battery electrodes, particularly in high-performance and long-cycle applications. Lead, despite its established use and cost-effectiveness in traditional lead-acid batteries, faces limitations in energy density and environmental toxicity that constrain future scalability. Emerging trends in energy storage favor superalloy-based electrodes for enhanced durability and sustainability, positioning them as the future benchmark over conventional lead materials.
Infographic: Superalloy vs Lead for Battery Electrode
 azmater.com
azmater.com