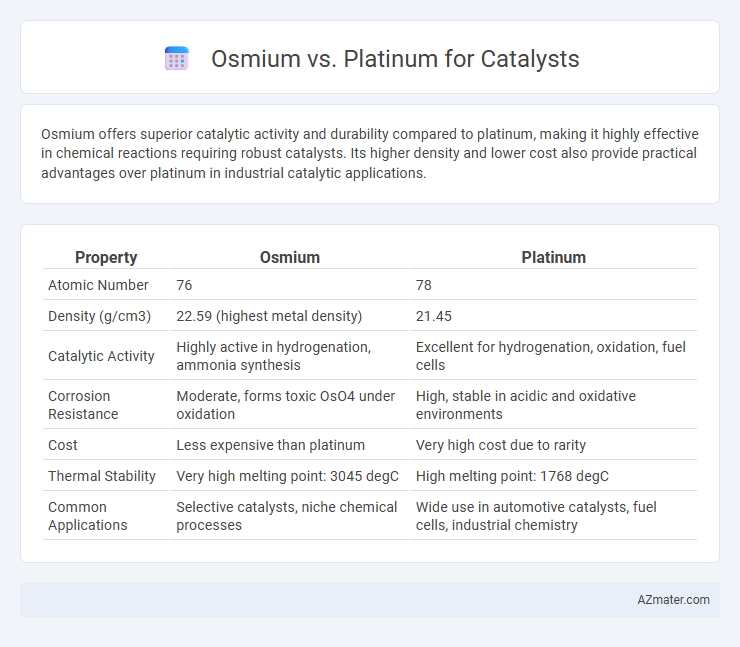
Osmium offers superior catalytic activity and durability compared to platinum, making it highly effective in chemical reactions requiring robust catalysts. Its higher density and lower cost also provide practical advantages over platinum in industrial catalytic applications.
Table of Comparison
| Property | Osmium | Platinum |
|---|---|---|
| Atomic Number | 76 | 78 |
| Density (g/cm3) | 22.59 (highest metal density) | 21.45 |
| Catalytic Activity | Highly active in hydrogenation, ammonia synthesis | Excellent for hydrogenation, oxidation, fuel cells |
| Corrosion Resistance | Moderate, forms toxic OsO4 under oxidation | High, stable in acidic and oxidative environments |
| Cost | Less expensive than platinum | Very high cost due to rarity |
| Thermal Stability | Very high melting point: 3045 degC | High melting point: 1768 degC |
| Common Applications | Selective catalysts, niche chemical processes | Wide use in automotive catalysts, fuel cells, industrial chemistry |
Introduction to Catalysts: Osmium vs Platinum
Osmium and platinum stand out as highly effective catalysts due to their unique electronic structures and surface properties, enabling accelerated chemical reactions. Platinum is widely preferred in catalytic converters and hydrogenation processes for its stability and resistance to poisoning, while osmium exhibits superior catalytic activity in oxidation reactions though it is less common owing to toxicity concerns. These distinctions make platinum more versatile in industrial applications whereas osmium finds niche uses where high catalytic efficiency is critical.
Chemical Properties and Reactivity
Osmium exhibits a higher density and greater electron density on its surface compared to platinum, leading to enhanced catalytic activity, particularly in oxidation reactions. Platinum is chemically more stable and less prone to oxidation, offering superior resistance to corrosion and longer catalyst life under harsh conditions. The distinct reactivity profiles make osmium suitable for specialized catalytic processes requiring strong oxidizing capabilities, while platinum is preferred for its robust stability and versatility in hydrogenation and fuel cell applications.
Catalytic Efficiency: Osmium Compared to Platinum
Osmium exhibits remarkable catalytic efficiency due to its high surface energy and excellent adsorption properties, often surpassing platinum in specific oxidation and hydrogenation reactions. While platinum remains widely used for its stability and versatility, osmium's unique electronic structure enables faster reaction rates and higher selectivity in catalysis under certain conditions. The denser atomic arrangement and greater electron donation capacity of osmium contribute to its enhanced catalytic performance in industrial applications such as fuel cells and chemical synthesis.
Industrial Applications and Use Cases
Osmium exhibits superior catalytic properties in industrial applications requiring high durability and resistance to corrosion, especially in oxygen reduction reactions and hydrogenation processes, outperforming platinum in harsh chemical environments. Platinum remains the preferred catalyst in automotive catalytic converters and fuel cells due to its exceptional activity and stability under moderate industrial conditions. Industries leverage osmium's robustness for chemical synthesis in aggressive media, while platinum dominates refining and pollution control technologies.
Cost and Availability of Osmium and Platinum
Osmium is significantly rarer and more expensive than platinum, with global reserves mostly concentrated in limited mining regions, leading to higher procurement costs and supply constraints for catalytic applications. Platinum, while also rare, has more established sources and a well-developed supply chain, making it comparatively more affordable and accessible for widespread catalytic use. The cost-effectiveness and availability of platinum often outweigh the performance advantages osmium might offer in catalytic reactions.
Environmental Impact and Sustainability
Osmium and platinum both serve as catalysts in industrial processes, but osmium's rarity and toxicity raise significant environmental concerns compared to platinum. Platinum, though still scarce, is more widely recycled and demonstrates better sustainability credentials with established recovery systems in automotive catalytic converters. The environmental impact of osmium is exacerbated by its potential to form highly toxic osmium tetroxide, making platinum a more environmentally favorable choice for catalyst applications.
Stability and Lifespan of Catalysts
Osmium catalysts exhibit remarkable thermal stability and resistance to oxidation, outperforming platinum in high-temperature and harsh chemical environments. Platinum catalysts, while highly effective, tend to degrade faster under prolonged exposure to reactive gases and elevated temperatures, shortening their operational lifespan. The superior durability of osmium-based catalysts makes them preferable for applications requiring extended catalyst longevity and consistent performance under extreme conditions.
Selectivity and Specificity in Catalytic Reactions
Osmium exhibits higher selectivity in catalytic reactions due to its unique electronic configuration and strong metal-support interactions, which enhance specific adsorption of reactants. Platinum, while widely used for its broad catalytic activity, often shows lower specificity, leading to more side reactions and reduced product purity. Studies reveal osmium-based catalysts outperform platinum in selective hydrogenation and oxidation processes, providing improved reaction control and yield.
Safety Considerations and Toxicity
Osmium and platinum exhibit distinct safety profiles as catalysts, with osmium posing higher toxicity risks due to its volatile osmium tetroxide, which can cause severe respiratory and skin irritation. Platinum catalysts are generally safer, exhibiting lower volatility and toxicity, though prolonged exposure may still trigger allergic reactions or sensitization. Proper handling protocols and protective equipment are essential when using osmium to mitigate its hazardous effects, whereas platinum requires standard safety measures.
Future Trends in Catalyst Development
Osmium and platinum both exhibit exceptional catalytic properties, with platinum currently dominating due to its superior durability and activity in fuel cells and automotive catalysts. Future trends in catalyst development emphasize enhancing osmium's catalytic efficiency through nanoscale engineering and alloy formation to reduce dependence on platinum's scarce reserves. Research is increasingly focused on optimizing osmium-platinum composites to achieve cost-effective, high-performance catalysts with improved resistance to poisoning and environmental degradation.
Infographic: Osmium vs Platinum for Catalyst
 azmater.com
azmater.com