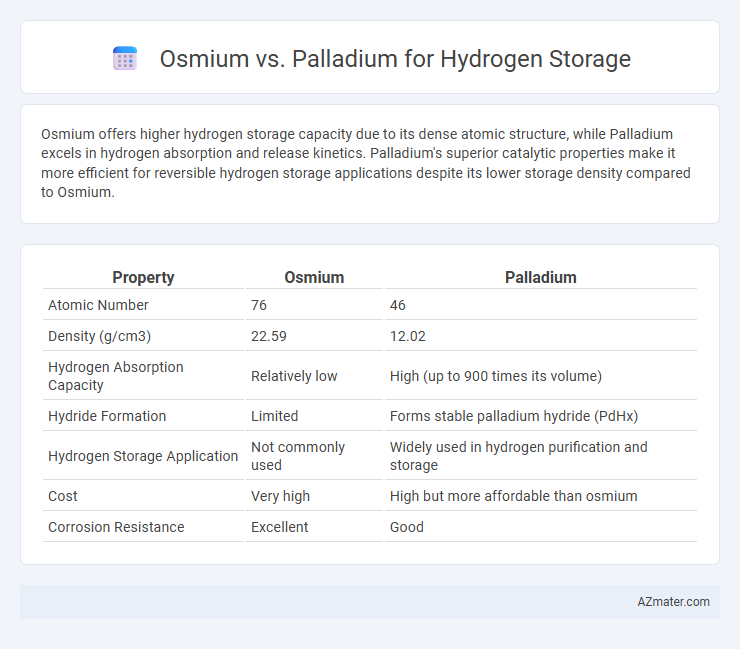
Osmium offers higher hydrogen storage capacity due to its dense atomic structure, while Palladium excels in hydrogen absorption and release kinetics. Palladium's superior catalytic properties make it more efficient for reversible hydrogen storage applications despite its lower storage density compared to Osmium.
Table of Comparison
| Property | Osmium | Palladium |
|---|---|---|
| Atomic Number | 76 | 46 |
| Density (g/cm3) | 22.59 | 12.02 |
| Hydrogen Absorption Capacity | Relatively low | High (up to 900 times its volume) |
| Hydride Formation | Limited | Forms stable palladium hydride (PdHx) |
| Hydrogen Storage Application | Not commonly used | Widely used in hydrogen purification and storage |
| Cost | Very high | High but more affordable than osmium |
| Corrosion Resistance | Excellent | Good |
Introduction to Hydrogen Storage Materials
Osmium and palladium are transition metals with distinct hydrogen storage capabilities due to their atomic structure and electron configurations. Palladium is widely recognized for its exceptional ability to absorb and store hydrogen at ambient conditions, forming palladium hydrides, which makes it a benchmark material in hydrogen storage research. Osmium, though less studied for hydrogen storage, offers potential due to its high density and lattice properties but typically requires higher pressures and temperatures to interact with hydrogen effectively.
Overview of Osmium and Palladium
Osmium and palladium are transition metals known for their unique properties in hydrogen storage applications, with palladium exhibiting exceptional hydrogen absorption capacity and reversible absorption-desorption cycles. Osmium, although less studied for hydrogen storage, features high density and strong metal-metal bonds, potentially influencing its hydrogen interaction dynamics. Palladium's face-centered cubic structure facilitates efficient hydrogen diffusion, making it a preferred material for hydrogen storage and purification technologies.
Physical and Chemical Properties Comparison
Osmium exhibits a higher density and melting point compared to palladium, which influences its hydrogen absorption capacity and storage stability under extreme conditions. Palladium's unique ability to absorb hydrogen up to 900 times its volume stems from its face-centered cubic crystal structure and atomic lattice that facilitates reversible hydrogen sorption. Chemically, palladium is more reactive with hydrogen, forming palladium hydride (PdHx) that provides efficient storage and release, whereas osmium's limited hydride formation reduces its effectiveness in hydrogen storage applications.
Hydrogen Absorption Mechanisms
Osmium and palladium exhibit distinct hydrogen absorption mechanisms critical for hydrogen storage applications. Palladium absorbs hydrogen through a reversible process involving the dissociation of H2 molecules into atomic hydrogen, which then diffuses into the metal lattice forming palladium hydride (PdHx) with high storage capacity. Osmium, on the other hand, demonstrates limited hydrogen solubility and absorption due to its denser atomic structure and weaker metal-hydrogen bonding, resulting in lower hydrogen uptake compared to palladium.
Storage Capacity: Osmium vs Palladium
Osmium exhibits a higher hydrogen storage capacity compared to palladium due to its denser atomic structure and greater ability to form hydrides. Palladium, while widely studied for hydrogen absorption, typically stores hydrogen up to 900 times its volume, but osmium can accommodate a higher hydrogen-to-metal ratio, enhancing volumetric storage efficiency. This makes osmium a promising candidate for advanced hydrogen storage applications where maximizing capacity is critical.
Efficiency and Kinetics of Hydrogen Release
Osmium exhibits superior hydrogen storage efficiency due to its higher hydrogen absorption capacity and stronger metal-hydrogen bonding, facilitating greater storage density compared to palladium. Palladium, however, demonstrates faster kinetics in hydrogen uptake and release, enabling quicker hydrogen diffusion and desorption rates. The trade-off between osmium's higher storage capacity and palladium's rapid hydrogen release kinetics is critical when optimizing materials for hydrogen storage applications.
Durability and Cycling Stability
Osmium exhibits superior durability under extreme conditions due to its high hardness and resistance to corrosion, making it highly stable during repeated hydrogen absorption and desorption cycles. Palladium offers excellent hydrogen permeability and moderate durability, but it tends to suffer from mechanical degradation and hydrogen embrittlement over prolonged cycling. In hydrogen storage applications, osmium provides enhanced cycling stability, while palladium requires surface treatments or alloying to improve long-term performance.
Cost and Economic Considerations
Osmium exhibits superior hydrogen absorption capacity but comes with significantly higher costs, making it less economically viable compared to palladium. Palladium, while offering moderate hydrogen storage efficiency, remains a preferred choice due to its lower price and wider availability. Cost-benefit analyses frequently favor palladium for scalable hydrogen storage applications given its balance between performance and affordability.
Environmental Impact and Sustainability
Osmium and palladium exhibit distinct environmental impacts in hydrogen storage applications due to their rarity and extraction processes; osmium's scarcity leads to higher ecological disruption compared to palladium. Palladium's wider availability and established recycling methods enhance its sustainability profile, reducing environmental footprint during production and end-of-life stages. Both metals require energy-intensive mining, but palladium's more efficient catalytic properties and recyclability position it as a relatively greener choice for sustainable hydrogen storage solutions.
Future Prospects and Research Directions
Osmium exhibits exceptional hydrogen absorption capacity and stability under high pressure, making it a promising candidate for advanced hydrogen storage systems, though its scarcity and cost limit large-scale application. Palladium offers efficient hydrogen uptake and release kinetics with well-established catalytic properties, positioning it as a versatile material in fuel cell technologies and hydrogen purification. Future research focuses on alloying strategies, nanoscale structuring, and hybrid material development to optimize storage capacity, enhance durability, and reduce economic barriers for both osmium and palladium in sustainable hydrogen energy solutions.
Infographic: Osmium vs Palladium for Hydrogen Storage
 azmater.com
azmater.com