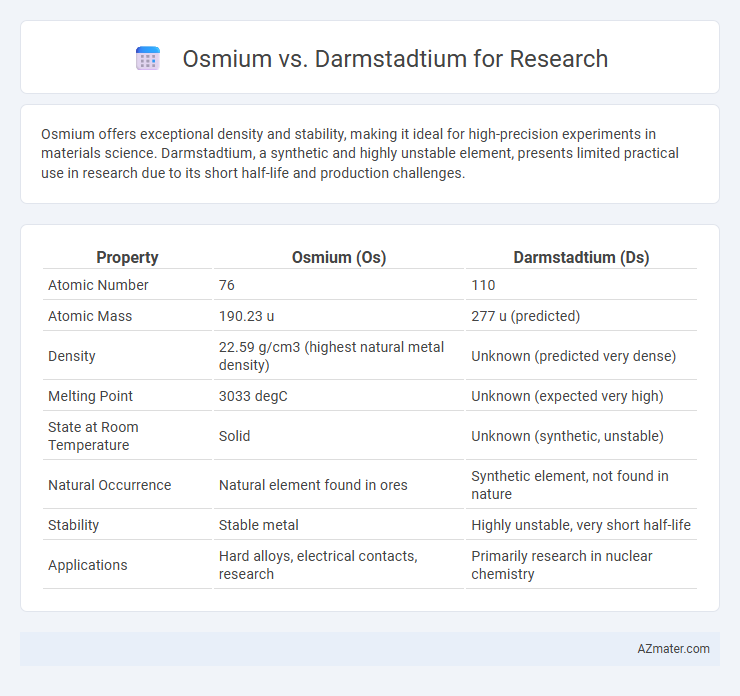
Osmium offers exceptional density and stability, making it ideal for high-precision experiments in materials science. Darmstadtium, a synthetic and highly unstable element, presents limited practical use in research due to its short half-life and production challenges.
Table of Comparison
| Property | Osmium (Os) | Darmstadtium (Ds) |
|---|---|---|
| Atomic Number | 76 | 110 |
| Atomic Mass | 190.23 u | 277 u (predicted) |
| Density | 22.59 g/cm3 (highest natural metal density) | Unknown (predicted very dense) |
| Melting Point | 3033 degC | Unknown (expected very high) |
| State at Room Temperature | Solid | Unknown (synthetic, unstable) |
| Natural Occurrence | Natural element found in ores | Synthetic element, not found in nature |
| Stability | Stable metal | Highly unstable, very short half-life |
| Applications | Hard alloys, electrical contacts, research | Primarily research in nuclear chemistry |
Introduction to Osmium and Darmstadtium
Osmium, a dense, blue-gray transition metal, is valued in research for its exceptional hardness, high melting point, and catalytic properties, making it crucial in materials science and chemistry studies. Darmstadtium, a synthetic element with atomic number 110, is highly unstable and primarily explored in nuclear physics to understand superheavy elements and their decay patterns. Research involving these elements highlights osmium's practical applications and darmstadtium's role in expanding knowledge of the periodic table's heaviest elements.
Elemental Properties Overview
Osmium, with an atomic number of 76, is a dense transition metal known for its high melting point (3045 K) and exceptional hardness, making it valuable in various industrial applications and material science research. Darmstadtium, atomic number 110, is a synthetic superheavy element with a very short half-life measured in milliseconds, limiting its research primarily to nuclear physics and theoretical chemistry. The stark contrast in stability and physical properties highlights osmium's suitability for applied research, whereas darmstadtium's exploration centers on understanding nuclear reactions and element formation.
Natural Occurrence and Synthesis
Osmium, a naturally occurring element with atomic number 76, is one of the densest metals found in the Earth's crust, primarily sourced from platinum ores, making it accessible for various research applications. Darmstadtium, atomic number 110, has no natural occurrence and is produced synthetically through nuclear fusion reactions in particle accelerators, limiting its availability for experimental studies. Research involving osmium benefits from its stability and natural abundance, whereas darmstadtium research focuses on understanding superheavy elements under highly controlled laboratory conditions.
Chemical Reactivity and Stability
Osmium exhibits remarkable chemical stability and low reactivity, making it valuable for catalysis and high-durability applications due to its dense atomic structure and robust electron configuration. Darmstadtium, a synthetic element with a very short half-life, displays extremely limited chemical reactivity, restricting practical research to theoretical modeling and rapid detection experiments in nuclear chemistry. The stark contrast in stability and reactivity between osmium and darmstadtium underscores osmium's suitability for experimental investigation while darmstadtium remains primarily of interest for fundamental nuclear science.
Applications in Scientific Research
Osmium is widely utilized in scientific research due to its exceptional density and hardness, making it ideal for applications like electron microscopy sample preparation and catalysts in chemical reactions. Darmstadtium, a synthetic and highly unstable element with a very short half-life, currently lacks practical applications in research beyond exploration of its nuclear properties and synthesis methods. The significant difference in stability and availability restricts darmstadtium's use, whereas osmium remains crucial in materials science and analytical chemistry.
Handling and Safety Considerations
Osmium, a dense and relatively stable transition metal, requires careful handling due to its toxic osmium tetroxide fumes formed upon oxidation, necessitating well-ventilated labs and protective equipment to avoid respiratory hazards. Darmstadtium, a synthetic element with extremely short half-lives, presents challenges primarily due to its radioactivity and limited availability, making direct handling virtually impossible and confining research largely to automated, remote systems with advanced shielding. Safety protocols emphasize minimizing exposure to osmium tetroxide's toxicity and radiation risks from darmstadtium decay, ensuring specialized containment and monitoring in high-security research facilities.
Analytical Techniques for Study
Osmium's dense atomic structure makes it ideal for advanced electron microscopy and X-ray diffraction techniques, providing detailed insights into material properties and crystallography. Darmstadtium, with its short half-life and synthetic origin, requires rapid, high-sensitivity mass spectrometry and nuclear spectroscopy for analysis before decay. Research on both elements benefits from cutting-edge spectroscopic methods, but osmium's stability allows for broader applications in analytical chemistry compared to the transient nature of darmstadtium studies.
Comparative Cost and Availability
Osmium, one of the densest naturally occurring elements, is relatively more available and affordable for research purposes compared to darmstadtium, which is a synthetic element produced only in trace amounts through particle accelerator experiments. The high cost and extreme rarity of darmstadtium limit its use primarily to theoretical and experimental nuclear research, while osmium's accessibility allows for broader applications in catalysis and material science. Researchers often prefer osmium when cost-effectiveness and availability are critical, whereas darmstadtium's study is constrained by production challenges and its short half-life.
Challenges in Experimental Use
Osmium, with its high density and relatively stable isotopes, presents challenges such as toxicity and difficulty in handling due to its brittleness and volatile osmium tetroxide formation during experiments. Darmstadtium, a synthetic element with extremely short half-lives measured in milliseconds, poses significant obstacles in experimental research because its rapid decay limits the ability to study its chemical and physical properties extensively. The scarcity and instability of darmstadtium require advanced detection techniques and rapid synthesis methods, contrasting with osmium's challenges related to material safety and durability in experimental setups.
Future Prospects in Advanced Research
Osmium, a dense transition metal with high durability and excellent catalytic properties, continues to play a significant role in material science and catalysis research, especially in developing advanced alloys and nanotechnology applications. Darmstadtium, a synthetic element with a very short half-life, remains primarily of academic interest with limited practical use, yet its position in the periodic table offers valuable insights for nuclear chemistry and superheavy element synthesis. Future prospects for osmium in advanced research center on expanding its applications in high-performance electronics and quantum materials, while darmstadtium's contributions lie in experimental nuclear physics and the exploration of element stability at extreme atomic numbers.
Infographic: Osmium vs Darmstadtium for Research
 azmater.com
azmater.com