Lead batteries offer high energy density and excellent recyclability, making them ideal for heavy-duty applications. Zinc batteries provide safer, more environmentally friendly alternatives with higher power output and longer cycle life in lightweight designs.
Table of Comparison
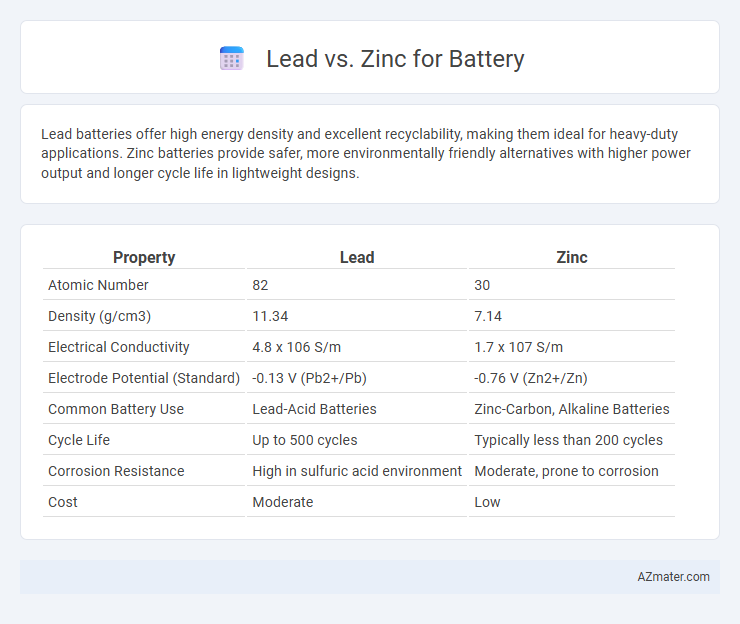
| Property | Lead | Zinc |
|---|---|---|
| Atomic Number | 82 | 30 |
| Density (g/cm3) | 11.34 | 7.14 |
| Electrical Conductivity | 4.8 x 106 S/m | 1.7 x 107 S/m |
| Electrode Potential (Standard) | -0.13 V (Pb2+/Pb) | -0.76 V (Zn2+/Zn) |
| Common Battery Use | Lead-Acid Batteries | Zinc-Carbon, Alkaline Batteries |
| Cycle Life | Up to 500 cycles | Typically less than 200 cycles |
| Corrosion Resistance | High in sulfuric acid environment | Moderate, prone to corrosion |
| Cost | Moderate | Low |
Introduction: The Role of Metals in Battery Technology
Lead and zinc play crucial roles in battery technology due to their distinct electrochemical properties and cost-effectiveness. Lead-acid batteries, known for high energy density and recyclability, dominate automotive and backup power applications, while zinc-based batteries offer benefits such as lightweight design and environmental friendliness. Innovations in zinc chemistry, including zinc-air and zinc-ion batteries, are driving advancements in sustainable energy storage solutions.
Overview of Lead and Zinc Batteries
Lead batteries, primarily lead-acid types, are widely used in automotive and backup power applications due to their high energy density and reliable cyclic stability. Zinc batteries, including zinc-air and zinc-ion variants, offer advantages in sustainability, cost-efficiency, and higher theoretical energy capacity but often face challenges with cycle life and commercialization. Both battery chemistries provide unique benefits, with lead batteries excelling in established large-scale energy storage systems, while zinc batteries show promising potential in emerging portable and grid applications.
Chemical Composition and Electrochemical Properties
Lead-acid batteries utilize lead dioxide (PbO2) as the positive electrode and sponge lead (Pb) as the negative electrode, while zinc-based batteries employ zinc (Zn) as the anode material paired with various cathode chemistries such as manganese dioxide or silver oxide. The electrochemical properties of lead-acid batteries, including a nominal voltage of 2.1 volts per cell and high discharge current capability, contrast with zinc batteries that typically offer higher energy density and a more negative electrode potential around -1.2 V vs. SHE, enabling enhanced voltage output and lighter weight. Chemical stability, corrosion resistance, and rechargeability are critical factors influencing performance and cycle life, with lead-acid chemistry favoring robustness and deep cycle applications, whereas zinc chemistries excel in lightweight and high energy density applications.
Energy Density: Lead vs. Zinc
Zinc batteries typically have a higher energy density compared to lead-acid batteries, offering more watt-hours per kilogram and enabling longer usage times for the same weight. Lead-acid batteries have energy densities around 30-40 Wh/kg, whereas zinc-based batteries can reach approximately 80-120 Wh/kg, making zinc a more efficient choice for applications requiring lightweight power sources. The improved energy density of zinc batteries supports advancements in portable electronics and electric vehicle technologies.
Lifecycle and Durability Comparison
Lead batteries provide a lifecycle of approximately 500 to 1,200 charge-discharge cycles, making them suitable for applications requiring moderate durability and reliable performance. Zinc batteries typically offer a shorter lifecycle, often ranging between 100 to 300 cycles, but benefit from lower weight and enhanced environmental sustainability. Durability in lead batteries excels due to robust corrosion resistance and established recycling infrastructure, while zinc batteries face challenges with material degradation and limited cycle stability.
Environmental Impact and Sustainability
Lead batteries contain toxic lead, posing significant environmental hazards during manufacturing, usage, and disposal, often requiring strict recycling protocols to prevent soil and water contamination. Zinc batteries utilize abundant, non-toxic zinc, offering a more sustainable alternative with lower ecological risks and easier end-of-life recycling. Zinc-based technologies generally support greater sustainability goals by minimizing heavy metal pollution and reducing reliance on hazardous materials.
Cost-Effectiveness Analysis
Lead batteries offer higher cost-effectiveness due to their lower initial investment and proven recyclability, resulting in reduced long-term expenses. Zinc batteries provide advantages in energy density and environmental safety but currently involve higher production costs and limited recycling infrastructure. Evaluating total lifecycle costs, including maintenance and replacement rates, positions lead batteries as more economically viable for large-scale, stationary energy storage applications.
Safety Concerns and Risk Factors
Lead batteries pose significant safety risks due to their toxicity and the potential for lead exposure, which can cause severe health issues, including neurological damage. Zinc batteries offer a safer alternative with lower toxicity and reduced environmental hazards, minimizing risks related to chemical spills and inhalation. Despite zinc's improved safety profile, both battery types require proper handling and disposal to prevent contamination and ensure user safety.
Real-World Applications and Use Cases
Lead-acid batteries dominate in automotive starting, lighting, and ignition systems due to their low cost and reliable performance under extreme temperatures. Zinc-based batteries, such as zinc-air and zinc-ion types, are increasingly utilized in renewable energy storage and wearable electronics for their high energy density and eco-friendly characteristics. Industrial forklifts and backup power systems often highlight lead batteries' robust cycling capability, while zinc batteries excel in lightweight, portable devices requiring long-lasting power.
Future Trends in Lead and Zinc Battery Innovation
Advancements in lead and zinc battery technology are driving significant improvements in energy density, charging speed, and cycle life, meeting the growing demand for sustainable energy storage solutions. Research focuses on enhancing lead-acid battery efficiency through nanomaterial electrodes and optimizing zinc-air batteries with solid-state electrolytes for safer, longer-lasting performance. Future trends highlight the integration of smart battery management systems and eco-friendly recycling processes, positioning lead and zinc batteries as competitive alternatives in grid storage and electric vehicle markets.
Infographic: Lead vs Zinc for Battery
 azmater.com
azmater.com