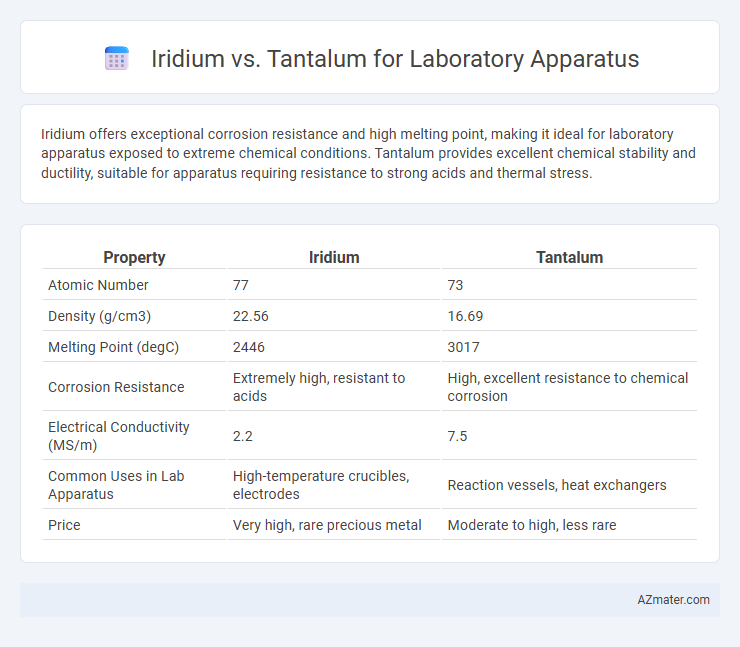
Iridium offers exceptional corrosion resistance and high melting point, making it ideal for laboratory apparatus exposed to extreme chemical conditions. Tantalum provides excellent chemical stability and ductility, suitable for apparatus requiring resistance to strong acids and thermal stress.
Table of Comparison
| Property | Iridium | Tantalum |
|---|---|---|
| Atomic Number | 77 | 73 |
| Density (g/cm3) | 22.56 | 16.69 |
| Melting Point (degC) | 2446 | 3017 |
| Corrosion Resistance | Extremely high, resistant to acids | High, excellent resistance to chemical corrosion |
| Electrical Conductivity (MS/m) | 2.2 | 7.5 |
| Common Uses in Lab Apparatus | High-temperature crucibles, electrodes | Reaction vessels, heat exchangers |
| Price | Very high, rare precious metal | Moderate to high, less rare |
Introduction to Iridium and Tantalum in Laboratory Apparatus
Iridium and tantalum are both highly valued in laboratory apparatus for their exceptional corrosion resistance and durability under extreme conditions. Iridium, a dense, platinum-group metal, excels in high-temperature environments and is often used in crucibles and electrodes due to its remarkable stability and resistance to oxidation. Tantalum, known for its excellent chemical inertness and high melting point, is commonly utilized in glass-lined reactors, heat exchangers, and vessels exposed to aggressive chemicals in laboratory settings.
Chemical Properties and Reactivity Comparison
Iridium exhibits exceptional chemical inertness, resisting oxidation and corrosion in most acids, making it highly suitable for laboratory apparatus exposed to harsh chemical environments. Tantalum, known for its strong resistance to attack by acids, especially sulfuric and hydrochloric acids, offers excellent durability but can be vulnerable to molten alkalis and hydrofluoric acid. The stable oxide layer of tantalum provides protective qualities, whereas iridium's robust metallic bonding results in superior chemical stability and lower reactivity under extreme lab conditions.
Mechanical Strength and Durability
Iridium exhibits exceptional mechanical strength and superior resistance to wear, making it ideal for laboratory apparatus subjected to extreme conditions. Tantalum offers excellent corrosion resistance but generally has lower hardness and mechanical durability compared to iridium. The combination of iridium's robustness and high melting point ensures long-lasting performance in precision instruments, surpassing tantalum in demanding mechanical applications.
Corrosion and Oxidation Resistance
Iridium offers superior corrosion resistance compared to tantalum, making it highly effective in harsh laboratory environments involving strong acids and oxidizing agents. Tantalum exhibits excellent resistance to a wide range of chemicals but can be susceptible to oxidation at elevated temperatures, unlike iridium which maintains stability under extreme oxidative conditions. For laboratory apparatus exposed to corrosive or oxidative stress, iridium ensures enhanced durability and longevity due to its robust chemical inertness.
Thermal Stability and Melting Points
Iridium exhibits superior thermal stability with a melting point of 2446degC, making it ideal for high-temperature laboratory apparatus that require durability under extreme heat. Tantalum has a lower melting point of 3017degC but excels in resisting chemical corrosion, beneficial for experiments involving reactive substances. Choosing iridium or tantalum depends on whether thermal endurance or chemical resistance is the critical factor for the laboratory application.
Cost and Availability of Iridium and Tantalum
Iridium is significantly more expensive and rarer than tantalum due to its scarcity and complex extraction process, making it less accessible for widespread laboratory apparatus use. Tantalum offers a more cost-effective and readily available alternative, with substantial global reserves primarily located in Australia, Brazil, and Africa. The pricing of tantalum remains relatively stable, driven by its higher abundance and diverse industrial applications compared to the niche demand for iridium in specialized equipment.
Weight Considerations and Density
Iridium, with a density of approximately 22.56 g/cm3, is significantly heavier than tantalum, which has a density of around 16.69 g/cm3. This higher density makes iridium lab apparatus more robust and resistant to wear, but also considerably heavier, impacting handling and transport. Tantalum's lighter weight offers easier maneuverability and lower overall mass in laboratory setups without compromising corrosion resistance in acidic environments.
Applications in Laboratory Settings
Iridium offers exceptional corrosion resistance and high melting point, making it ideal for crucibles and electrodes in high-temperature laboratory experiments. Tantalum is preferred for its excellent chemical inertness and resistance to acid attack, often used in reaction vessels and piping systems handling aggressive chemicals. Both metals are crucial in laboratory apparatus where durability and chemical stability under extreme conditions are required.
Environmental and Safety Implications
Iridium and tantalum exhibit distinct environmental and safety implications in laboratory apparatus applications. Iridium, being rarer and more chemically inert, poses fewer risks of corrosion and leaching, reducing environmental contamination but raises concerns due to limited recyclability and high extraction energy demands. Tantalum's excellent biocompatibility and corrosion resistance enhance laboratory safety, yet mining activities often involve conflict minerals and generate significant ecological disruption, necessitating careful sourcing and waste management strategies.
Conclusion: Choosing the Right Material
Iridium offers exceptional corrosion resistance and high melting point, making it ideal for harsh laboratory environments requiring durability and chemical inertness. Tantalum excels in resisting a broad range of acids and provides excellent thermal conductivity, suitable for applications involving aggressive chemical reactions at moderate temperatures. Selecting the right material depends on the specific chemical exposure and temperature conditions, with iridium preferred for extreme environments and tantalum favored for cost-effective resistance to strong acids.
Infographic: Iridium vs Tantalum for Laboratory Apparatus
 azmater.com
azmater.com