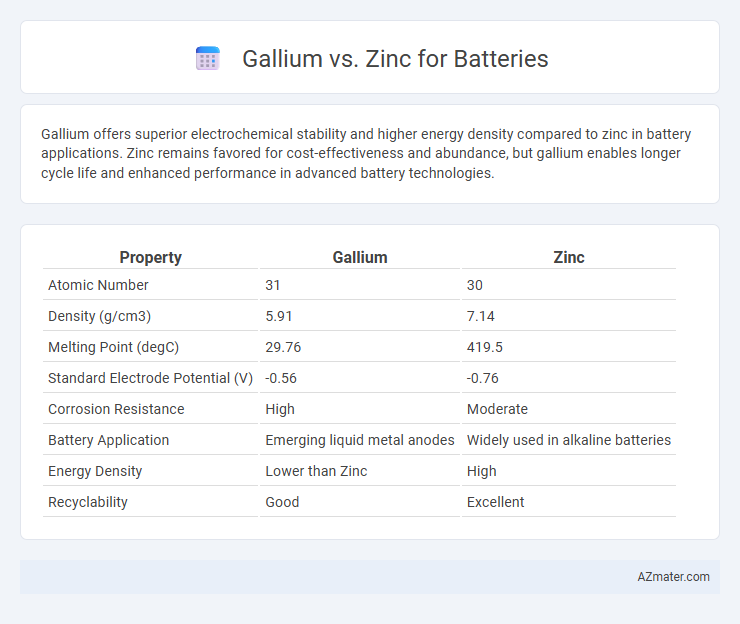
Gallium offers superior electrochemical stability and higher energy density compared to zinc in battery applications. Zinc remains favored for cost-effectiveness and abundance, but gallium enables longer cycle life and enhanced performance in advanced battery technologies.
Table of Comparison
| Property | Gallium | Zinc |
|---|---|---|
| Atomic Number | 31 | 30 |
| Density (g/cm3) | 5.91 | 7.14 |
| Melting Point (degC) | 29.76 | 419.5 |
| Standard Electrode Potential (V) | -0.56 | -0.76 |
| Corrosion Resistance | High | Moderate |
| Battery Application | Emerging liquid metal anodes | Widely used in alkaline batteries |
| Energy Density | Lower than Zinc | High |
| Recyclability | Good | Excellent |
Introduction: Gallium and Zinc in Modern Batteries
Gallium and zinc are emerging materials in advanced battery technology, each offering unique electrochemical properties that influence performance. Zinc batteries provide high energy density and are cost-effective with excellent safety profiles, making them suitable for large-scale energy storage. Gallium, often used in alloy form, enhances battery stability and conductivity, demonstrating potential in flexible and wearable energy devices.
Chemical Properties: Gallium vs Zinc
Gallium exhibits a low melting point of 29.76degC and resists corrosion through oxide layer formation, while zinc has a higher melting point of 419.5degC and actively forms zinc oxide in alkaline batteries. Gallium's ability to wet and alloy with other metals enhances its use in liquid metal batteries, contrasting with zinc's high electrochemical reactivity and role as an anode material in conventional alkaline and zinc-air batteries. The redox potential of zinc (-0.76 V) differs significantly from gallium's (+0.53 V), affecting their respective voltage outputs and energy densities in battery applications.
Battery Technologies Using Gallium
Battery technologies using gallium leverage its ability to stabilize metal anodes and prevent dendrite formation, enhancing battery lifespan and safety compared to traditional zinc-based batteries. Gallium's high melting point and excellent conductivity contribute to improved performance in liquid metal batteries, offering higher energy density and faster charge-discharge cycles. Research into gallium alloys demonstrates promising results for grid storage applications, where efficiency and durability are critical factors.
Zinc-Based Battery Applications
Zinc-based batteries offer cost-effective and environmentally friendly energy storage solutions with high safety due to zinc's non-toxic and abundant nature. Incorporating gallium into zinc anodes enhances battery performance by reducing dendrite formation, which improves cycle life and charge efficiency. Zinc-gallium alloys enable advanced applications in grid storage, electric vehicles, and portable electronics by combining zinc's affordability with gallium's stability benefits.
Energy Density Comparison
Gallium batteries exhibit higher volumetric energy density compared to zinc batteries, making them suitable for compact energy storage applications. Zinc batteries boast superior gravimetric energy density, offering lightweight solutions with decent capacity but lower overall energy per unit volume. The choice between gallium and zinc battery chemistries depends on the specific application requirements for energy density, size constraints, and weight considerations.
Cost and Resource Availability
Gallium batteries tend to be more expensive due to the limited availability and higher extraction costs of gallium compared to zinc, which is abundant and inexpensive. Zinc's widespread availability and lower market price make it a cost-effective choice for large-scale battery production. The economic feasibility of zinc-based batteries benefits from established supply chains and sustainable sourcing, contrasting with the relatively niche and costly gallium resources.
Environmental Impact: Gallium vs Zinc Batteries
Gallium batteries produce lower toxic waste compared to traditional zinc batteries, reducing environmental contamination during disposal. Zinc batteries rely on abundant materials but can cause soil and water pollution due to zinc leaching, impacting ecosystems negatively. Gallium's higher recyclability and stable electrochemical properties contribute to a more sustainable and eco-friendly battery technology.
Performance and Efficiency Metrics
Gallium batteries exhibit higher energy density and longer cycle life compared to zinc-based batteries, making them superior in performance for high-demand applications. Gallium's excellent conductivity and corrosion resistance contribute to more efficient charge-discharge cycles, resulting in enhanced overall battery efficiency. Zinc batteries, while cost-effective and environmentally friendly, typically suffer from lower energy density and faster capacity degradation, impacting their long-term performance metrics.
Innovations in Gallium and Zinc Batteries
Innovations in gallium batteries focus on leveraging gallium's low melting point and high conductivity to enhance battery flexibility and safety, enabling next-generation flexible and wearable electronic devices. Zinc batteries benefit from breakthroughs in aqueous zinc-ion technology, improving energy density, cycle life, and eco-friendliness for large-scale energy storage applications. Recent research emphasizes gallium's potential in liquid metal electrodes and zinc's advancements in dendrite suppression, driving significant improvements in battery efficiency and durability.
Future Prospects and Industry Trends
Gallium's unique properties, such as its low melting point and high conductivity, offer promising advancements for next-generation batteries with enhanced flexibility and efficiency compared to traditional zinc-based cells. Zinc remains a cost-effective and environmentally friendly option widely used in large-scale applications, but its performance limitations in energy density drive research toward gallium-infused alloys and hybrid technologies. Industry trends indicate growing investment in gallium battery development for wearable electronics and grid storage, where improved durability and recyclability align with sustainable energy goals.
Infographic: Gallium vs Zinc for Battery
 azmater.com
azmater.com