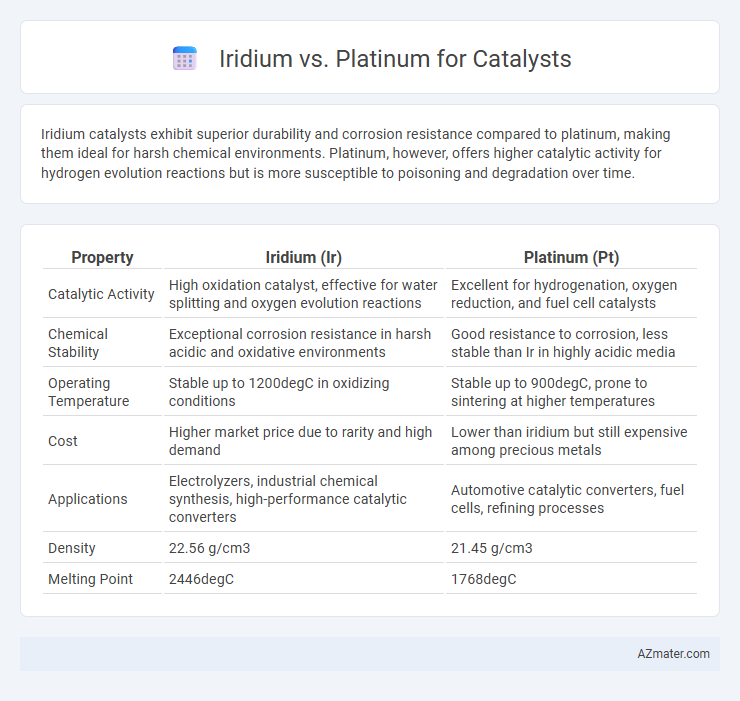
Iridium catalysts exhibit superior durability and corrosion resistance compared to platinum, making them ideal for harsh chemical environments. Platinum, however, offers higher catalytic activity for hydrogen evolution reactions but is more susceptible to poisoning and degradation over time.
Table of Comparison
| Property | Iridium (Ir) | Platinum (Pt) |
|---|---|---|
| Catalytic Activity | High oxidation catalyst, effective for water splitting and oxygen evolution reactions | Excellent for hydrogenation, oxygen reduction, and fuel cell catalysts |
| Chemical Stability | Exceptional corrosion resistance in harsh acidic and oxidative environments | Good resistance to corrosion, less stable than Ir in highly acidic media |
| Operating Temperature | Stable up to 1200degC in oxidizing conditions | Stable up to 900degC, prone to sintering at higher temperatures |
| Cost | Higher market price due to rarity and high demand | Lower than iridium but still expensive among precious metals |
| Applications | Electrolyzers, industrial chemical synthesis, high-performance catalytic converters | Automotive catalytic converters, fuel cells, refining processes |
| Density | 22.56 g/cm3 | 21.45 g/cm3 |
| Melting Point | 2446degC | 1768degC |
Introduction to Catalysts: Iridium vs Platinum
Iridium and platinum are among the most effective catalysts used in electrochemical reactions, particularly in water splitting and fuel cells. Iridium exhibits superior stability and corrosion resistance under acidic and high-potential conditions, making it ideal for oxygen evolution reactions in harsh environments. Platinum, while highly active for hydrogen evolution reactions, offers excellent catalytic efficiency but is less stable than iridium in highly oxidative conditions, influencing catalyst selection based on application demands.
Chemical Properties and Reactivity
Iridium exhibits higher resistance to corrosion and oxidation compared to platinum, making it more durable as a catalyst under harsh chemical environments. Platinum offers superior catalytic activity in hydrogenation and fuel cell reactions due to its optimal electronic configuration and surface adsorption properties. The differential reactivity stems from iridium's strong metal-metal bonds and platinum's ability to facilitate electron transfer and intermediate adsorption efficiently.
Catalytic Efficiency Comparison
Iridium catalysts exhibit higher catalytic efficiency in oxygen evolution reactions (OER) due to their superior activity and stability under acidic conditions compared to platinum. While platinum demonstrates excellent catalytic performance in hydrogen evolution reactions (HER), its efficiency declines in OER environments, where iridium's ability to maintain active sites and resist corrosion is advantageous. Iridium's unique electronic structure and optimal adsorption energy for reaction intermediates contribute to its enhanced catalytic efficiency, making it a preferred choice in energy conversion applications such as water splitting.
Cost and Economic Considerations
Iridium catalysts typically incur higher costs due to limited supply and complex extraction processes, impacting large-scale industrial applications. Platinum offers a relatively lower price and greater availability, making it more economically viable for widespread catalytic use. However, the choice between Iridium and Platinum catalysts also depends on specific reaction efficiency and lifespan, influencing overall cost-effectiveness.
Availability and Resource Sustainability
Iridium is significantly rarer than platinum, with annual production of only about 3 metric tons compared to platinum's 180 metric tons, which limits its availability for large-scale catalysis applications. Platinum's more abundant supply contributes to better resource sustainability and potential for recycling, making it a preferred choice in catalytic converters and fuel cells. However, iridium's exceptional corrosion resistance ensures longer catalyst lifespans, balancing scarcity concerns with durability in harsh environments.
Industrial Applications
Iridium catalysts exhibit superior resistance to corrosion and high temperature stability, making them ideal for industrial applications such as water electrolysis and hydrogenation reactions. Platinum catalysts offer exceptional activity and selectivity in fuel cells and catalytic converters due to their well-established catalytic properties. Industrial processes benefit from iridium's durability in harsh environments, while platinum remains preferred for reactions requiring optimal electron transfer and oxidation resistance.
Environmental Impact and Safety
Iridium catalysts demonstrate superior environmental benefits due to their higher stability, reducing the generation of hazardous waste compared to platinum catalysts, which tend to degrade faster under harsh conditions. Iridium's lower toxicity and resistance to leaching minimize environmental contamination and human exposure risks during industrial processes. Platinum, while highly effective, poses greater environmental concerns due to its susceptibility to poisoning and higher consumption rates, leading to increased mining impacts and safety challenges in catalyst recycling.
Durability and Longevity
Iridium catalysts exhibit superior durability and longevity compared to platinum, especially in harsh oxidative and acidic environments due to their higher resistance to corrosion and dissolution. Platinum catalysts, while efficient in catalysis, tend to degrade faster under prolonged electrochemical operation, leading to reduced lifespan and performance stability. The enhanced stability of iridium makes it the preferred choice for applications requiring long-term catalyst durability, such as in water electrolysis and fuel cells.
Recent Innovations in Catalyst Design
Recent innovations in catalyst design highlight iridium's superior catalytic activity and stability compared to platinum, especially in oxygen evolution reactions (OER) crucial for water splitting applications. Advanced doping techniques and nanostructuring have enhanced iridium's electron conductivity and surface area, significantly boosting its efficiency while reducing precious metal loading. Emerging research emphasizes tailored iridium-platinum alloys that synergize the excellent corrosion resistance of iridium with platinum's hydrogen adsorption properties, optimizing catalysts for fuel cells and electrolyzers.
Future Outlook for Iridium and Platinum Catalysts
Iridium catalysts demonstrate growing potential in hydrogen evolution and oxygen reduction reactions due to their exceptional stability and activity under harsh conditions, positioning them as key materials for next-generation water-splitting technologies. Platinum catalysts continue to dominate fuel cell applications with high efficiency and well-established manufacturing infrastructure, but face challenges related to cost and scarcity that drive research towards alloying and alternative supports. Future outlook for iridium involves innovation in dispersion and support materials to reduce loading while enhancing durability, whereas platinum developments prioritize cost reduction and expanded use in electrolyzers and automotive fuel cells.
Infographic: Iridium vs Platinum for Catalyst
 azmater.com
azmater.com