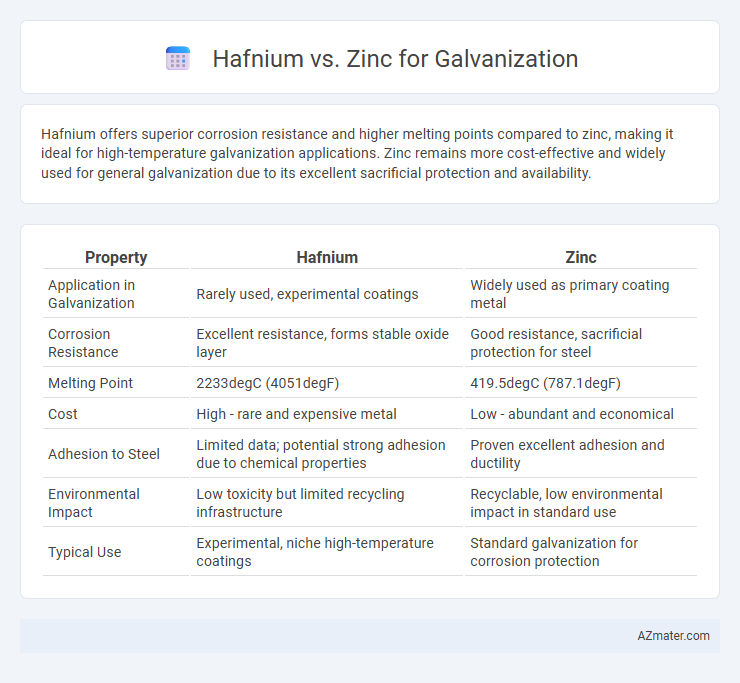
Hafnium offers superior corrosion resistance and higher melting points compared to zinc, making it ideal for high-temperature galvanization applications. Zinc remains more cost-effective and widely used for general galvanization due to its excellent sacrificial protection and availability.
Table of Comparison
| Property | Hafnium | Zinc |
|---|---|---|
| Application in Galvanization | Rarely used, experimental coatings | Widely used as primary coating metal |
| Corrosion Resistance | Excellent resistance, forms stable oxide layer | Good resistance, sacrificial protection for steel |
| Melting Point | 2233degC (4051degF) | 419.5degC (787.1degF) |
| Cost | High - rare and expensive metal | Low - abundant and economical |
| Adhesion to Steel | Limited data; potential strong adhesion due to chemical properties | Proven excellent adhesion and ductility |
| Environmental Impact | Low toxicity but limited recycling infrastructure | Recyclable, low environmental impact in standard use |
| Typical Use | Experimental, niche high-temperature coatings | Standard galvanization for corrosion protection |
Overview of Galvanization: Purpose and Methods
Galvanization serves as a protective process to prevent steel and iron corrosion by applying a metal coating, typically zinc, which offers sacrificial anodic protection. Zinc remains the preferred choice due to its cost-effectiveness, excellent corrosion resistance, and ability to form a durable barrier layer through hot-dip galvanizing or electro-galvanizing methods. Hafnium, while possessing strong corrosion resistance and high melting point, is rarely used in galvanization because of its scarcity and cost, limiting its practicality compared to zinc in industrial corrosion protection applications.
Chemical Properties of Hafnium and Zinc
Hafnium exhibits high chemical stability with excellent resistance to corrosion and oxidation, making it suitable for protective coatings in extreme environments. Zinc, widely used in galvanization, has moderate corrosion resistance due to its ability to form a stable zinc oxide layer that protects underlying metals. The key difference lies in zinc's sacrificial anodic behavior, which prevents steel corrosion, whereas hafnium's chemical inertness offers a more robust, albeit less commonly utilized, protective barrier.
Historical Use of Zinc in Galvanization
Zinc has been the predominant metal used in galvanization since the 19th century due to its excellent corrosion resistance and cost-effectiveness. Its historical application in protecting steel structures, such as bridges and automotive bodies, has proven its durability by forming a stable zinc oxide layer that prevents rust. Although hafnium offers superior corrosion resistance and higher melting point, its rarity and expense limit its practical use compared to zinc's widespread availability and established industrial infrastructure.
Emerging Interest in Hafnium for Coatings
Hafnium is gaining attention as a potential alternative to zinc for galvanization due to its superior corrosion resistance and higher melting point, which enhances durability in harsh environments. Emerging research highlights hafnium-based coatings' ability to form dense, stable oxide layers that offer improved protection against oxidation and wear compared to conventional zinc coatings. This growing interest in hafnium stems from its promising performance in aerospace and nuclear industries, signaling potential for advanced, long-lasting galvanization applications.
Corrosion Resistance: Hafnium vs Zinc
Hafnium exhibits superior corrosion resistance compared to zinc due to its ability to form dense, stable oxide layers that protect metal substrates from oxidation and environmental degradation. Zinc provides effective galvanic protection by sacrificing itself to corrode preferentially, but its protection can diminish over time as the zinc layer depletes. In harsh or high-temperature environments, hafnium coatings maintain longer-lasting corrosion resistance, making them more suitable for critical applications requiring enhanced durability.
Cost Comparison: Hafnium and Zinc for Industrial Use
Zinc remains the dominant choice for galvanization due to its significantly lower cost, averaging around $2.50 per kilogram compared to Hafnium's price, which can exceed $400 per kilogram. The substantial cost difference makes Zinc more economically viable for large-scale industrial applications, despite Hafnium's superior corrosion resistance and higher melting point. Industries prioritize Zinc for cost efficiency, reserving Hafnium for specialized uses where performance justifies the premium expense.
Environmental Impact of Hafnium and Zinc Galvanization
Hafnium galvanization poses significant environmental concerns due to its rarity and energy-intensive extraction process, leading to higher carbon emissions compared to zinc. Zinc galvanization is more environmentally favorable, as zinc is abundant, recyclable, and involves less energy consumption during production, reducing its overall ecological footprint. Managing zinc runoff is critical to prevent soil and water contamination, highlighting the need for proper waste treatment in galvanized steel applications.
Performance in Different Environmental Conditions
Hafnium exhibits superior corrosion resistance compared to zinc when used for galvanization in harsh environments, including marine and industrial atmospheres. Zinc performs well in moderate conditions but tends to degrade faster under acidic or highly saline exposures due to its lower oxidation stability. Hafnium's dense oxide layer provides enhanced durability, extending the protective lifespan of galvanized steel in extreme weather and chemical settings.
Technological Advances in Hafnium-Based Galvanization
Hafnium-based galvanization offers superior corrosion resistance and enhanced thermal stability compared to traditional zinc coatings, making it ideal for high-performance industrial applications. Recent technological advances in hafnium deposition techniques, such as atomic layer deposition (ALD), enable ultra-thin, uniform coatings that significantly improve substrate durability and lifespan. These innovations drive the adoption of hafnium in sectors requiring extreme protection, including aerospace and nuclear industries, where zinc galvanization falls short.
Future Prospects: Which Metal Leads in Galvanization?
Hafnium's corrosion resistance and high melting point position it as a promising candidate for advanced galvanization applications, especially in extreme environments or next-generation electronics. Zinc remains the dominant metal for galvanization due to its cost-effectiveness, excellent sacrificial anode properties, and widespread industrial adoption. Future prospects favor zinc for large-scale infrastructure corrosion protection, while hafnium could lead in specialized, high-performance coatings where durability and resistance to harsh conditions are critical.
Infographic: Hafnium vs Zinc for Galvanization
 azmater.com
azmater.com